Difference between revisions of "Part:BBa K346003"
| (10 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K346003 short</partinfo> | <partinfo>BBa_K346003 short</partinfo> | ||
| − | This part is a | + | This part is a translational unit for expression of mercury metal binding peptide which was engineered from MerR. It was designed to be expressed in the cytosol but when fused with the periplasm protein DsbA or the membrane protein Lpp-OmpA, it will be expressed and translocated to the periplasm and membrane. In short, this part functions as the basic mercury binding protein in our project. |
| Line 10: | Line 10: | ||
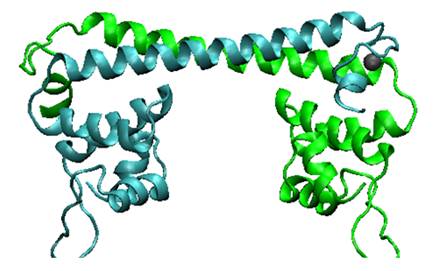
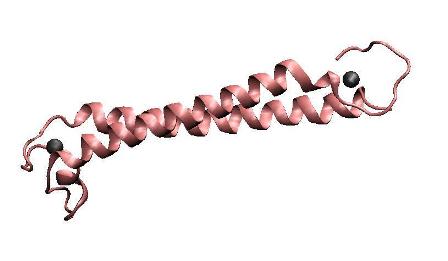
| − | MerR, the mercury- | + | MerR, the mercury-responsive transcription factor(figure on the left), acts as an effective mercury capturer in aquatic environment. However, as an regulator, over-expression of MerR in bacteria may lead to some unpredictable side effect. Earlier work suggested that the constructed peptide only consisting of the metal binding domain can form a stable dimer with its mercury binding affinity remaining.The DNA binding domain and metal binding domain can function individually.Based on all these above and careful structure analysis of MerR via 3D structure modeling, we directly tandemed two copies of metal binding domain of MerR together, to implement a mercury metal binding peptide (MBP) as is shown in the figure on the right. |
| − | [[Image:MerR.gif]] [[Image:mbp2.jpg]] | + | [[Image:MerR v2.gif]] [[Image:mbp2.jpg]] |
| − | The figure shows the structure of MerR and MBP. The left figure is MerR, the mercury | + | The figure shows the structure of MerR and MBP. The left figure is MerR, the mercury sensitive transcription factor. The right figure shows the predicted structure of resulted metal binding peptide. Mercury ions are indicated as black balls in metal binding pockets. |
| − | == | + | |
| + | == Results: == | ||
---- | ---- | ||
| − | |||
| − | |||
| − | + | '''Expression of proteins''' | |
| + | |||
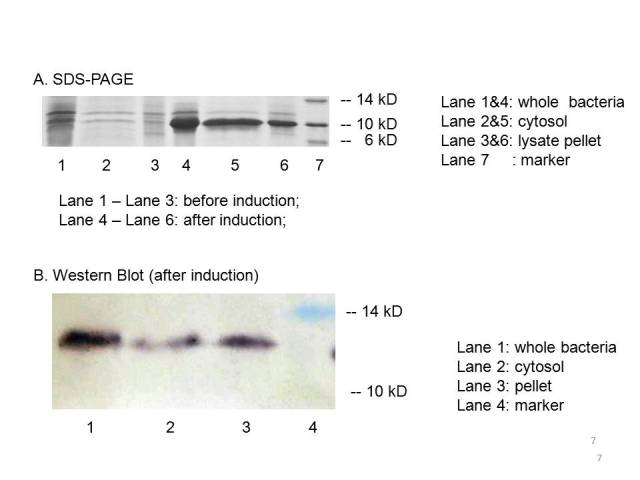
| + | The plasmid PET21a-MBP is transferred to E.coli strain BL21, which can generate T7 polymerase when induced with IPTG. Both induced bacteria and uninduced bacteria(as control) are prepared to make seperate sample of the cytosol, the periplasm and the membrane. The SDS-page and Western blot of the samples showed that compared with the uninduced bacteria, the induced group expressed an protein at the proper place with the size of ~10kD which consists with the predicted size, indicating that the engineered MBP can be expressed in the cytosol. | ||
| + | |||
| + | [[Image:result of MBP.jpg]] | ||
| + | |||
| + | We can detected overexpression band at about 10 kD, which is similar to MBP. Then we fused a his-tag to our target protein and conducted western blot to further verify their expression. Positive band of the expected molecular weight can be detected in the cytosol, which confirm expression and localization of the target protein. We can also indicate from the SDS-PAGE that there exist a large number of MBPs in cytosol which are ready to bind mercury ions. | ||
| + | |||
| + | |||
| + | '''Function test''' | ||
| + | |||
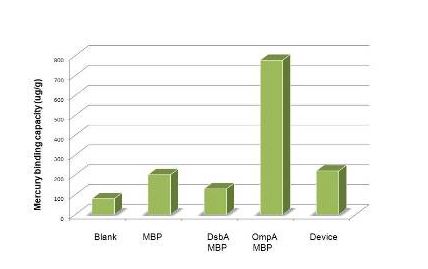
| + | Having made sure that the protein can express normally in the cytosol, the function tests were carried out with ICP-AES. To test the efficiency of mercury absorption of MBP in different concentration of mercury, the concentration gradient from 10^-8M to 10^-6M was set. The results are shown in figure 3. Our experiment shows that the efficiency of MBP increases with the the mercury concentration. Additionally, MBP can bind Hg(II) with high efficiency and sensitivity from the concentration of 10^-7 M(not show in the figure). | ||
| + | |||
| + | [[Image:dfadsf v2.jpg]] | ||
| + | |||
| + | Figure 3: Overnight culture was diluted 1:100 and cultured until OD600=0.6-1.0. We then resuspended the culture with 1% PBS after centrifuging it. Samples for ICP-AES were made following the protocol(See more about this, please visit our wiki.) | ||
| − | |||
| − | |||
| − | |||
Latest revision as of 04:30, 28 October 2010
RBS(B0032)+MBP(mercury metal binding peptide engineered from MerR)
This part is a translational unit for expression of mercury metal binding peptide which was engineered from MerR. It was designed to be expressed in the cytosol but when fused with the periplasm protein DsbA or the membrane protein Lpp-OmpA, it will be expressed and translocated to the periplasm and membrane. In short, this part functions as the basic mercury binding protein in our project.
Description
MerR, the mercury-responsive transcription factor(figure on the left), acts as an effective mercury capturer in aquatic environment. However, as an regulator, over-expression of MerR in bacteria may lead to some unpredictable side effect. Earlier work suggested that the constructed peptide only consisting of the metal binding domain can form a stable dimer with its mercury binding affinity remaining.The DNA binding domain and metal binding domain can function individually.Based on all these above and careful structure analysis of MerR via 3D structure modeling, we directly tandemed two copies of metal binding domain of MerR together, to implement a mercury metal binding peptide (MBP) as is shown in the figure on the right.
The figure shows the structure of MerR and MBP. The left figure is MerR, the mercury sensitive transcription factor. The right figure shows the predicted structure of resulted metal binding peptide. Mercury ions are indicated as black balls in metal binding pockets.
Results:
Expression of proteins
The plasmid PET21a-MBP is transferred to E.coli strain BL21, which can generate T7 polymerase when induced with IPTG. Both induced bacteria and uninduced bacteria(as control) are prepared to make seperate sample of the cytosol, the periplasm and the membrane. The SDS-page and Western blot of the samples showed that compared with the uninduced bacteria, the induced group expressed an protein at the proper place with the size of ~10kD which consists with the predicted size, indicating that the engineered MBP can be expressed in the cytosol.
We can detected overexpression band at about 10 kD, which is similar to MBP. Then we fused a his-tag to our target protein and conducted western blot to further verify their expression. Positive band of the expected molecular weight can be detected in the cytosol, which confirm expression and localization of the target protein. We can also indicate from the SDS-PAGE that there exist a large number of MBPs in cytosol which are ready to bind mercury ions.
Function test
Having made sure that the protein can express normally in the cytosol, the function tests were carried out with ICP-AES. To test the efficiency of mercury absorption of MBP in different concentration of mercury, the concentration gradient from 10^-8M to 10^-6M was set. The results are shown in figure 3. Our experiment shows that the efficiency of MBP increases with the the mercury concentration. Additionally, MBP can bind Hg(II) with high efficiency and sensitivity from the concentration of 10^-7 M(not show in the figure).
Figure 3: Overnight culture was diluted 1:100 and cultured until OD600=0.6-1.0. We then resuspended the culture with 1% PBS after centrifuging it. Samples for ICP-AES were made following the protocol(See more about this, please visit our wiki.)
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 184
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]