Difference between revisions of "Part:BBa K346002"
| (17 intermediate revisions by 4 users not shown) | |||
| Line 2: | Line 2: | ||
| − | This part, PmerT, is a promoter from Tn21 mercury resistance (mer) operon. The mer operon | + | This part, PmerT, is a promoter from Tn21 mercury resistance (mer) operon. The regulatory region of mer operon consists of two tightly overlapped, divergently oriented promoters – Pr and Ptpad.(Park, Wireman et al. 1992). Pr is the promoter of the regulatory protein gene, ''merR'', and Ptpcad is for the transcription of the structure gene – ''merPTAD''. Both of them are called ''merOP'' as a whole. |
---- | ---- | ||
| − | [[Image:MerR-dimer.jpg]] | + | [[Image:MerR-dimer.jpg|center]] |
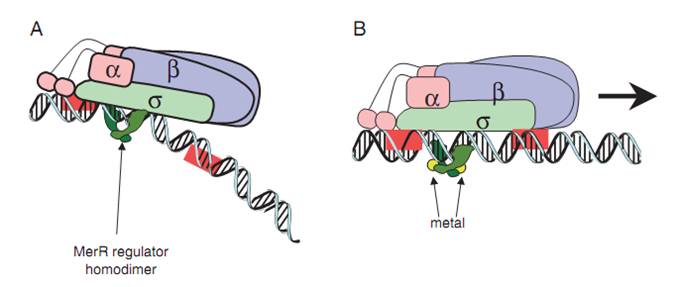
| − | (A) The dimeric MerR regulator binds to the operator region of the promoter and recruits RNA polymerase, forming a ternary complex. Transcription is slightly repressed because the apo-MerR regulator dimer has bent the promoter DNA such that RNA polymerase does not contact it properly. (B) Upon binding the cognate metal ions (shown as cyan circles) the metallated MerR homodimer causes a realignment of the promoter such that RNA polymerase contacts the -35 and -10 sequences leading to open complex formation and transcription. | + | '''Fig.1. The mechanism of MerR mediated transcriptional activation.''' (A) The dimeric MerR regulator binds to the operator region of the promoter and recruits RNA polymerase, forming a ternary complex. Transcription is slightly repressed because the apo-MerR regulator dimer has bent the promoter DNA such that RNA polymerase does not contact it properly. (B) Upon binding the cognate metal ions (shown as cyan circles) the metallated MerR homodimer causes a realignment of the promoter such that RNA polymerase contacts the -35 and -10 sequences leading to open complex formation and transcription. Adapted from Brown et al. |
| + | ---- | ||
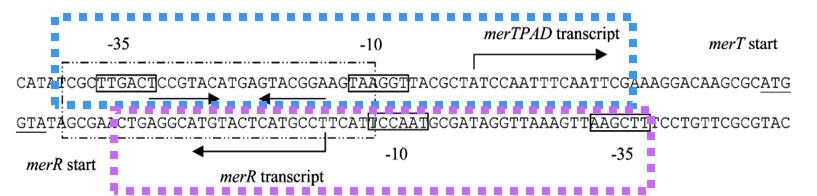
| − | + | The key sequence for MerR’s binding is a region of interrupted dyad symmetry (19bp) located between the -35 and -10 haxamers of Ptpcad (The top strand). And the structure of Pr (botton strand) is similar to Ptpcad in a divergent orientation. The -10 hexamers of Ptpcad and Pr actually overlap by four bases. When the apo-MerR dimer bind to the dyad symmetrical operator DNA between the -35 and – 10 elements of mercury inducible promoter, PmerT, which has a unusually long spacer of 19 bp for MerR to bind on, the binding of RNA polymerase is inhibited(Fig.2). The Hg-bound MerR can result in an a structural distortion of PmerT, allowing the RNA polymerase contacts to be made, leading to the expression of downstream genes. This model of transcription activation indicates that the apo-MerR and Hg-bound MerR have a competing relationship. The threshold of PmerT is controlled by the expression level of MerR. As a consequence, the sensitivity of Hg(II) in cell can be manipulated. | |
| − | + | [[Image:PmerT111.jpg|center]] | |
| − | + | '''Fig.2. DNA sequence of the Tn21 mer operon promoter region.''' The MerR binding site on PmerT is marked by a box. The -35 and -10 regions for both PmerR and PmerTPAD are marked with boxes, and the dyad symmetrical DNA sequence that MerR recognizes and binds to is marked with arrows under the DNA sequence. The divergently oriented promoters are marked by blue box and purple box, respectively. | |
| + | ---- | ||
| − | ''' | + | '''For more information, please check 'Experience' and our wiki!''' |
| + | ---- | ||
| + | ===BEAS_China 2019: Experimental Characterization=== | ||
| + | <html> | ||
| + | <p><b>BEAS_China 2019 used BBa_K346002 as the promoter of GFP in our basic mercury sensor design: J23 family-merR-pMerT-sfGFP-terminator. </b>This sensor has a constitutive promoter (J23 family) that drives the expression of an mercury receptor MerR, which would de-repress its cognate promoter merR on murcury binding and trigger the expression of a reporter gene, gfp. </p> | ||
| + | <div style="text-align: center;"> | ||
| + | <img src="https://2019.igem.org/wiki/images/b/b1/T--BEAS_China--Description_Basic_sensor_Principle.png" alt="" width="700"> | ||
| + | <h6 style="text-align:center">Figure 3: The scheme of basic sensor design. </h6> | ||
| + | </div> | ||
| + | </html> | ||
| − | + | <html> | |
| − | + | <p>We select three constitutive promoters of varying strengths from iGEM promoter library (Fig. 4A). The sensors were then compared under various HgNO3 induction conditions (Fig. 4B). The results showed that the weaker the promoter (that is, the lower the MerR receptor concentration), the more sensitive and higher the dynamic range of the sensor.</p> | |
| + | <div style="text-align: center;"> | ||
| + | <img src="https://2019.igem.org/wiki/images/6/6c/T--BEAS_China--Demonstration_Fig_1a_%26_1b.png" alt="" width="700"> | ||
| + | <h6 style="text-align:center">Figure 4: <strong>A</strong> Different constitutively J23 family promoter measured strength (Data source: iGEM) <strong>B</strong> Tuning mercury receptor merR’s intracellular density by varying the strength of J23 prmoter </h6> | ||
| + | </div> | ||
| − | + | <p>We fitted the sensors’ dose–response curves to a Hill function-based biochemical model to describe their input-output relationships. (Fig 5 and Table 1) </p> | |
| − | + | <ul> | |
| − | + | <li> | |
| − | + | <p>The Hill constant EC50 is the inducer concentration that provokes half-maximal activation of a sensor; EC50 is negatively correlated with sensitivity.</p> | |
| − | + | </li> | |
| − | + | <li> | |
| − | + | <p>KTop is the sensor’s maximum output expression level; KTop is positively correlated with output amplitude.</p> | |
| − | + | </li> | |
| − | + | </ul> | |
| − | == | + | <div style="text-align: center;"> |
| − | + | <img src="https://2019.igem.org/wiki/images/d/d0/T--BEAS_China--Demonstration_Fig_2.png" alt="" width="700"> | |
| − | + | <h6 style="text-align:center">Figure 5: The equation used to fit the sensors’ dose–response curves to a Hill function based biochemical model to describe their input-GFPput relationships | |
| − | + | </h6> | |
| + | <img src="https://2019.igem.org/wiki/images/2/2a/T--BEAS_China--Demonstration_Table1.png" alt="" width="700"> | ||
| + | <h6 style="text-align:center">Table 1: Best fits for the characterized response of the various sensors circuits in this study | ||
| + | </h6> | ||
| + | </div> | ||
| + | <p>Here, EC50 showed a 2.7-fold decrease and KTop showed a 3.5-fold increase from high to low MerR levels (Fig. 6a & 6b ), confirming that the mercury sensor’s sensitivity and output amplitude were both increased while the MerR intracellular concentration was decreased. </p> | ||
| + | <div style="text-align: center;"> | ||
| + | <img src="https://2019.igem.org/wiki/images/5/56/T--BEAS_China--Demonstration_Fig_3A_%26_3B.png" alt="" width="700"> | ||
| + | <h6 style="text-align:center">Figure 6: The maximum output (KTop) and EC50 of the sensor’s dose response against the relevant intracellular MerR concentrations </h6> | ||
| + | </div> | ||
| + | </html> | ||
Latest revision as of 14:38, 21 October 2019
PmerT promoter (mercury-responsive)
This part, PmerT, is a promoter from Tn21 mercury resistance (mer) operon. The regulatory region of mer operon consists of two tightly overlapped, divergently oriented promoters – Pr and Ptpad.(Park, Wireman et al. 1992). Pr is the promoter of the regulatory protein gene, merR, and Ptpcad is for the transcription of the structure gene – merPTAD. Both of them are called merOP as a whole.
Fig.1. The mechanism of MerR mediated transcriptional activation. (A) The dimeric MerR regulator binds to the operator region of the promoter and recruits RNA polymerase, forming a ternary complex. Transcription is slightly repressed because the apo-MerR regulator dimer has bent the promoter DNA such that RNA polymerase does not contact it properly. (B) Upon binding the cognate metal ions (shown as cyan circles) the metallated MerR homodimer causes a realignment of the promoter such that RNA polymerase contacts the -35 and -10 sequences leading to open complex formation and transcription. Adapted from Brown et al.
The key sequence for MerR’s binding is a region of interrupted dyad symmetry (19bp) located between the -35 and -10 haxamers of Ptpcad (The top strand). And the structure of Pr (botton strand) is similar to Ptpcad in a divergent orientation. The -10 hexamers of Ptpcad and Pr actually overlap by four bases. When the apo-MerR dimer bind to the dyad symmetrical operator DNA between the -35 and – 10 elements of mercury inducible promoter, PmerT, which has a unusually long spacer of 19 bp for MerR to bind on, the binding of RNA polymerase is inhibited(Fig.2). The Hg-bound MerR can result in an a structural distortion of PmerT, allowing the RNA polymerase contacts to be made, leading to the expression of downstream genes. This model of transcription activation indicates that the apo-MerR and Hg-bound MerR have a competing relationship. The threshold of PmerT is controlled by the expression level of MerR. As a consequence, the sensitivity of Hg(II) in cell can be manipulated.
Fig.2. DNA sequence of the Tn21 mer operon promoter region. The MerR binding site on PmerT is marked by a box. The -35 and -10 regions for both PmerR and PmerTPAD are marked with boxes, and the dyad symmetrical DNA sequence that MerR recognizes and binds to is marked with arrows under the DNA sequence. The divergently oriented promoters are marked by blue box and purple box, respectively.
For more information, please check 'Experience' and our wiki!
BEAS_China 2019: Experimental Characterization
BEAS_China 2019 used BBa_K346002 as the promoter of GFP in our basic mercury sensor design: J23 family-merR-pMerT-sfGFP-terminator. This sensor has a constitutive promoter (J23 family) that drives the expression of an mercury receptor MerR, which would de-repress its cognate promoter merR on murcury binding and trigger the expression of a reporter gene, gfp.

Figure 3: The scheme of basic sensor design.
We select three constitutive promoters of varying strengths from iGEM promoter library (Fig. 4A). The sensors were then compared under various HgNO3 induction conditions (Fig. 4B). The results showed that the weaker the promoter (that is, the lower the MerR receptor concentration), the more sensitive and higher the dynamic range of the sensor.

Figure 4: A Different constitutively J23 family promoter measured strength (Data source: iGEM) B Tuning mercury receptor merR’s intracellular density by varying the strength of J23 prmoter
We fitted the sensors’ dose–response curves to a Hill function-based biochemical model to describe their input-output relationships. (Fig 5 and Table 1)
-
The Hill constant EC50 is the inducer concentration that provokes half-maximal activation of a sensor; EC50 is negatively correlated with sensitivity.
-
KTop is the sensor’s maximum output expression level; KTop is positively correlated with output amplitude.

Figure 5: The equation used to fit the sensors’ dose–response curves to a Hill function based biochemical model to describe their input-GFPput relationships

Table 1: Best fits for the characterized response of the various sensors circuits in this study
Here, EC50 showed a 2.7-fold decrease and KTop showed a 3.5-fold increase from high to low MerR levels (Fig. 6a & 6b ), confirming that the mercury sensor’s sensitivity and output amplitude were both increased while the MerR intracellular concentration was decreased.

Figure 6: The maximum output (KTop) and EC50 of the sensor’s dose response against the relevant intracellular MerR concentrations
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]