Difference between revisions of "Part:BBa K404201"
(→Modification of the Viral Capsid of the AAV2 using Viral Bricks) |
|||
| (57 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K404201 short</partinfo> | <partinfo>BBa_K404201 short</partinfo> | ||
| + | {| style="color:black" cellpadding="6" cellspacing="1" border="2" align="left" | ||
| + | ! colspan="2" style="background:#66bbff;"|[https://parts.igem.org/Part:BBa_K404201 ViralBrick-453-BAP] | ||
| + | |- | ||
| + | |'''BioBrick Nr.''' | ||
| + | |[https://parts.igem.org/Part:BBa_K404201 BBa_K404201] | ||
| + | |- | ||
| + | |'''RFC standard''' | ||
| + | |[https://parts.igem.org/Help:Assembly_standard_25 RFC 25] | ||
| + | |- | ||
| + | |'''Requirement''' | ||
| + | |pSB1C3<br> | ||
| + | |- | ||
| + | |'''Source''' | ||
| + | | | ||
| + | |- | ||
| + | |'''Submitted by''' | ||
| + | |[http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] | ||
| + | |} | ||
| + | <br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/> | ||
| + | <br><b>The Biotinylation Acceptor peptide motif, ready for insertion into the 453 loop</b> | ||
| + | |||
| + | [[Image:Freiburg10_ViralBrick-logo-453-BAP.png|thumb|center|480px]]<br> | ||
| + | |||
| + | |||
{| style="color:black" cellpadding="6" cellspacing="1" border="2" align="right" | {| style="color:black" cellpadding="6" cellspacing="1" border="2" align="right" | ||
! colspan="2" style="background:#66bbff;"|[https://parts.igem.org/Part:BBa_K404201 ViralBrick-453-BAP] | ! colspan="2" style="background:#66bbff;"|[https://parts.igem.org/Part:BBa_K404201 ViralBrick-453-BAP] | ||
| Line 12: | Line 36: | ||
|[https://parts.igem.org/Help:Assembly_standard_25 RFC 25][https://parts.igem.org/Help:Assembly_standard_10 RFC 10] | |[https://parts.igem.org/Help:Assembly_standard_25 RFC 25][https://parts.igem.org/Help:Assembly_standard_10 RFC 10] | ||
|- | |- | ||
| − | |''' | + | |'''Requirement''' |
| − | |[https://parts.igem.org/wiki/index.php?title=Part:BBa_K404200 pSB1C3_VCK] | + | |backbone without <br> |
| + | SspI, SalI, BamHI and PvuII<br> | ||
| + | (e.g. [https://parts.igem.org/wiki/index.php?title=Part:BBa_K404200 pSB1C3_VCK]) | ||
|- | |- | ||
|'''Source''' | |'''Source''' | ||
| Line 22: | Line 48: | ||
|} | |} | ||
<br> | <br> | ||
| − | All capsid coding parts (e.g. [https://parts.igem.org/Part:BBa_K404001 (AAV2)-RepVP123]) of the Virus Construction Kit designed by the iGEM Team Freiburg contain single cutting restriction sites on the side of the two major surface exposed | + | All capsid coding parts (e.g. [https://parts.igem.org/Part:BBa_K404001 (AAV2)-RepVP123]) of the Virus Construction Kit designed by the iGEM Team Freiburg contain single cutting restriction sites on the side of the sequences coding for the two major surface exposed loops. <br> |
Using these restriction sites it is possible to insert functional motifs into the coding sequence for the viral capsid.<br><br> | Using these restriction sites it is possible to insert functional motifs into the coding sequence for the viral capsid.<br><br> | ||
This BioBrick contains one of these functional motifs fanked by bilateral linkers and the viral sequences that code for the loop.<br> | This BioBrick contains one of these functional motifs fanked by bilateral linkers and the viral sequences that code for the loop.<br> | ||
| − | This category of BioBricks was termed ViralBrick to clarify that cloning does not function with the usual iGEM RFC restriction sites but with the mentioned ViralBrick restriction | + | This category of BioBricks was termed ViralBrick to clarify that cloning does not function with the usual iGEM RFC restriction sites but with the mentioned ViralBrick restriction sites.<br><br> |
| + | |||
| + | The four restriction sites for the ViralBrick insertion were designed in a way that the amino acid sequence of the viral capsid could be absolutely conserved. This was advisable because even slight changes in the viral capsid lead to drastically changed interactions with cellular surface receptors. For this reason we decided to insert these restriction sites in stead of using BioBrick assembly that would define at least two amino acids in the viral loop. | ||
| − | |||
<br><br><br><br> | <br><br><br><br> | ||
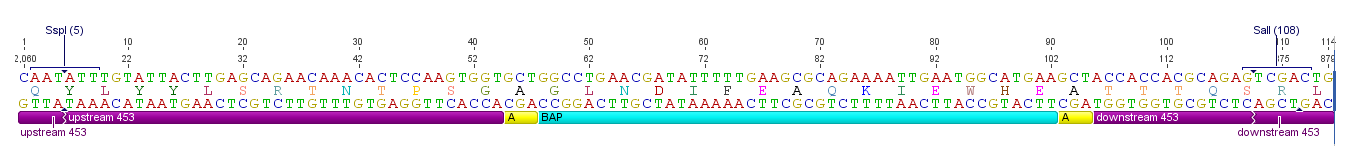
[[image:Freiburg10_ViralBrick-453-BAP.png|thumb|920px|Schematic representation of the BioBrick with all relevant annotations]] | [[image:Freiburg10_ViralBrick-453-BAP.png|thumb|920px|Schematic representation of the BioBrick with all relevant annotations]] | ||
| − | ==== | + | ===Restriction sites=== |
| − | + | ||
| − | + | ||
| − | + | <table border="1" style="float:right; width:480px; height:auto;> | |
| + | <tr> | ||
| + | <th><b>Restriction site</b></th> | ||
| + | <th><b>Enzyme</b></th> | ||
| + | <th><b>Recognition sequence</b></th> | ||
| + | <th><b>Activity</b></th> | ||
| + | <th><b>Temp.</b></th> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>upstream 453</td> | ||
| + | <td>[http://www.neb.com/nebecomm/products/productR3132.asp SspI-HF]</td> | ||
| + | <td>[[image:Freiburg10_recognition site SspI-HF.Gif]]</td> | ||
| + | <td>25;100;0;100</td> | ||
| + | <td>37°C</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>downstream 453</td> | ||
| + | <td>[http://www.neb.com/nebecomm/products/productR3138.asp SalI-HF]</td> | ||
| + | <td>[[image:Freiburg10_recognition site SalI-HF.Gif]]</td> | ||
| + | <td>10;100;100;100</td> | ||
| + | <td>37°C</td> | ||
| − | + | <tr> | |
| − | < | + | </table> |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | <table border="1"> | + | <table border="1" style="float:right; width:480px; height:auto;> |
<tr> | <tr> | ||
<th><b>Restriction site</b></th> | <th><b>Restriction site</b></th> | ||
| − | <th><b>Enzyme | + | <th><b>Enzyme</b></th> |
<th><b>Recognition sequence</b></th> | <th><b>Recognition sequence</b></th> | ||
| − | <th><b>Activity | + | <th><b>Activity</b></th> |
| − | <th><b> | + | <th><b>Temp.</b></th> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 87: | Line 125: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | </table> | + | </table><html><br><br><br><br> |
| + | |||
| + | <!---ViralBrick BAP---> | ||
| + | <center><h1>Specific Biotinylation via ViralBrick: The Biotinylation Acceptor Peptide</h1></center> | ||
| + | <div style="width:965px; height:400px; "> | ||
| + | <div style="float:left; width:460px; height:auto; margin: 0px 5px 0px 5px; text-align:justify;"> | ||
| + | The BAP (Biotinylation Acceptor Peptide) that we included in our Virus Construction Kit is a 15 amino acid long peptide identified by Schatz J., 1993 in an library screening approach and published under the number #85. This peptide with the sequence 5' - GLNDIFEAQKIEWHE - 3' contains a central lysine that is specifically biotinylated by the prokaryotic enzyme biotin holenzyme synthetase, encoded in the BirA gene of E. coli. Specific biotinylation of this peptide sequence can be performed in vivo by contransfecting a plasmid with the BirA gene as described for the AAV in Arnold et al.; 2006 or by an in vitro coupling approach using the purified Escherichia coli enzyme biotin ligase (BirA). <br> | ||
| + | The purified BirA biotin ligase that was kindly provided from Avidity.com | ||
| + | <h3>References:</h3> | ||
| + | </div> | ||
| + | <div style="float:right; width:480px; height:auto; "><img src="https://static.igem.org/mediawiki/parts/a/a9/Freiburg10_ViralBrick_motif_BAP.png" width="460" | ||
| + | height="auto"/></div></div><br><br><br><br> | ||
| + | |||
| + | <!---ViralBrick His---> | ||
| + | <center><h1>IMAC purification via Viral Brick: The Histidin Affinity Tag</h1></center> | ||
| + | <div style="width:965px; height:400px; "> | ||
| + | <div style="float:left; width:460px; height:auto; margin: 0px 5px 0px 5px; text-align:justify;"> | ||
| + | Protein tagging via Histidine Tags is a widely used method for protein purification: Multiple histidine residues (most commonly: Six) are being fused tot he end of the targeting protein.<br> | ||
| + | The high binding affinity of Histidine towards metal is being exploited for the purification of proteins via the so called „Immobilized Metal Ion Affinity Chromatography“ (IMAC): Multiple histidine residues (most commonly: Six) are being fused to the end of the targeting protein. A cell extract containing the recombinant protein ist then applied to a collumn containing immobilized Ni2+-Ions. The His-tags covalently bind the Ni-Ions while other cellular proteins can be washed oft he collumn. The purified proteins can then be eluted with Imidazol, which displaces the histidine residues.(Smith et al. 1988), (Hoffmann & Roeder 1991)<br> | ||
| + | Since the aim behind engineering therapeutic AAV vectors is a safe administration to human patients, it is important to consider a convenient way of purifying the virus particles. Contamination by cellular proteins could cause toxic side effects or a strong immune response. Koerber et al. have first inserted a His-tag into a surface-exposed loop at amino acid position 587 in the Cap protein and successfully purified recombinant virsuses using IMAC (Koerber et al. 2007). For our Virus Construction Kit, we provide the His-tag motif in the ViralBrick standard, allowing for an easy insertion into the 453 and/or 587 loop. If the modified capsid bearing a His-tag is being cotransfected with a wild type capsid for the production of mosaic viruses, IMAC helps to not only purify the produced viral particles but also to enrich particles which actually contain the modified proteins. <br> | ||
| + | |||
| + | |||
| + | <h3>References:</h3> | ||
| + | </div> | ||
| + | <div style="float:right; width:480px; height:auto; "><img src="https://static.igem.org/mediawiki/parts/5/59/Freiburg10_ViralBrick_motif_His.png" width="460" | ||
| + | height="auto"/></div></div><br><br><br><br> | ||
| + | |||
| + | <!--- ViralBrick RGD---> | ||
| + | <center><h1>Targetingpeptides via ViralBrick: The RGD Motif</h1></center> | ||
| + | <div style="width:965px; height:400px; "> | ||
| + | <div style="float:left; width:460px; height:auto; margin: 0px 5px 0px 5px; text-align:justify;"> | ||
| + | |||
| + | <h3>References:</h3> | ||
| + | </div> | ||
| + | <div style="float:right; width:480px; height:auto; "><img src="https://static.igem.org/mediawiki/parts/f/f6/Freiburg10_ViralBrick_motif_RGD.png" width="460" | ||
| + | height="auto"/></div></div> | ||
| + | |||
| + | <!---ViralBrick Z34C---> | ||
| + | <center><h1>Coupling Antibodies to the Viral Surface via ViralBrick: The Z34C Motif</h1></center> | ||
| + | <div style="width:965px; height:600px; "> | ||
| + | <div style="float:left; width:460px; height:auto; margin: 0px 5px 0px 5px; text-align:justify;"> | ||
| + | |||
| + | The idea of this targeting approach is to utilise a minimized fragment of the <a href=http://www.ncbi.nlm.nih.gov/protein/153106>Staphylococcal Protein A</a> that was first described in Staphylococcus aureus. This gram-positive bacteria has evolved the 508 amino acid long protein A that has a high affinity for the Fc-domain of antibodies to protect itself from the immune systeme. Binding to the constant region of the antibodies is accomplished by the <a href=http://www.ncbi.nlm.nih.gov/protein/1Q2NA>Z-Domain</a> of Protein A that is 58-59 amino acids long, has alone a high affinity (Kd= 14,9 nM) for the antibodies and a three-helix bundle structure. In <a href=http://www.ncbi.nlm.nih.gov/pubmed/8650153>[Braisted & Wells; 1996]</a> the authors reduced the secundary structure to an two-helix bundle. This size reduction has lead to an drastic reduction of the affinity for IgG (>10^5 fold) which could be recovered by 13 amino acid exchanges resulting in a 38 amino acid long peptide with an satisfying affinity for IgG (Kd = 185 nM) termed <a href=http://www.ncbi.nlm.nih.gov/protein/1ZDAA>Z38</a>. This binding domain was subsequently improoved in <a href=http://www.ncbi.nlm.nih.gov/pubmed/9294166>[Starovasnik et al.; 1997]</a> by the insertion of a disulfide bridge connecting the ends of the helices leading to the binding domain <a href=http://www.ncbi.nlm.nih.gov/protein/1ZDCA>Z34C</a> which shows an increased affinity for IgG (Kd = 20 nM).<br> | ||
| + | This engineered antibody binding domain of 34 amino acids was then inserted into capsids of different viral vectors amongst others also the AAV. In <a href=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC155067>[Ried et al.; 2002]</a> the <a href=http://www.ncbi.nlm.nih.gov/protein/1ZDCA>Z34C</a> domain was inserted at position 587 into the capsid of the AAV resulting in viral vector that can be targeted to different target cells without genetic engineering. This targeting approach was then improved in <a href=http://www.ncbi.nlm.nih.gov/pubmed/15922956>[Gigout et al.; 2005]</a> by the creation of mosaic vectors that contain only ~25% of recombinant VP-Proteins what resulted in 4 to 5 orders of magnitude more infectiosity compared to all-mutant viruses. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <h3>References:</h3> | ||
| + | <li><a href=http://www.ncbi.nlm.nih.gov/pubmed/8650153>[Braisted & Wells; 1996]</a></li> | ||
| + | <li><a href=http://www.ncbi.nlm.nih.gov/pubmed/9294166>[Starovasnik et al.; 1997]</a></li> | ||
| + | <li><a href=http://www.ncbi.nlm.nih.gov/pubmed/11932421>[Ried et al.; 2002]</a></li> | ||
| + | <li><a href=http://www.ncbi.nlm.nih.gov/pubmed/15922956>[Gigout et al.; 2005]</a></li> | ||
| + | </div> | ||
| + | <div style="float:right; width:480px; height:auto; "><img src="https://static.igem.org/mediawiki/parts/9/98/Freiburg10_ViralBrick_motif_Z34C.png" width="460" | ||
| + | height="auto"/></div> | ||
| + | <div style="float:right; width:480px; height:auto; "><img src="https://static.igem.org/mediawiki/2010/e/e9/Freiburg10_Evolution_of_Z34C.png" width="460" | ||
| + | height="auto"/></div> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <!---Risk assessment---> | ||
| + | <center><h1>Risk assessment of the Adeno-associated Virus</h1></center> | ||
| + | <div style="width:965px; height:300px; "> | ||
| + | <div style="float:left; width:500px; height:auto; margin: 0px 5px 0px 5px; text-align:justify;"> | ||
| + | <div style="float:right; width:480px; height:auto; "><img src="https://static.igem.org/mediawiki/2010/2/26/Freiburg10_DangerI.png" width="320" | ||
| + | height="auto"/></div> | ||
| + | Viral Systems based on the Adeno-associated Virus of the serotype 2 are to handle under Biosafety level 1 according to the german <a href=http://bundesrecht.juris.de/gentg/index.html>Act on Genetic Engineering</a> the </html>[[Media:Freiburg10_specification of the ZKBS on working with the AAV.pdf| specification of the ZKBS on working with the AAV]]<html>. In the United States, the legal regulations are comparable according to the National Institutes of Health (NIH) which classifies the AVV-2 as BSL-1 in the <a | ||
| + | href="http://oba.od.nih.gov/oba/rac/guidelines_02/Appendix_B.htm">Appendix | ||
| + | B</a>. This general classification is restricted to experiments that use the ITRs (Inverted Terminal Repeats) of the AAV2 and do not contain hazardous gene sequences on the vector plasmid.<br> | ||
| + | Please consider special provisions of law in your country before using parts that contribute to the production of genetically modified viral vectors. | ||
| + | </div> | ||
| − | |||
| − | + | </div> | |
| − | + | ||
| − | + | ||
| − | + | ||
| + | </html> | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Latest revision as of 02:16, 28 October 2010
ViralBrick-453-BAP
| ViralBrick-453-BAP | |
|---|---|
| BioBrick Nr. | BBa_K404201 |
| RFC standard | RFC 25 |
| Requirement | pSB1C3 |
| Source | |
| Submitted by | [http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] |
The Biotinylation Acceptor peptide motif, ready for insertion into the 453 loop
| ViralBrick-453-BAP | |
|---|---|
| BioBrick Nr. | BBa_K404201 |
| RFC standard | RFC 25RFC 10 |
| Requirement | backbone without SspI, SalI, BamHI and PvuII |
| Source | synthetic |
| Submitted by | [http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] |
All capsid coding parts (e.g. (AAV2)-RepVP123) of the Virus Construction Kit designed by the iGEM Team Freiburg contain single cutting restriction sites on the side of the sequences coding for the two major surface exposed loops.
Using these restriction sites it is possible to insert functional motifs into the coding sequence for the viral capsid.
This BioBrick contains one of these functional motifs fanked by bilateral linkers and the viral sequences that code for the loop.
This category of BioBricks was termed ViralBrick to clarify that cloning does not function with the usual iGEM RFC restriction sites but with the mentioned ViralBrick restriction sites.
The four restriction sites for the ViralBrick insertion were designed in a way that the amino acid sequence of the viral capsid could be absolutely conserved. This was advisable because even slight changes in the viral capsid lead to drastically changed interactions with cellular surface receptors. For this reason we decided to insert these restriction sites in stead of using BioBrick assembly that would define at least two amino acids in the viral loop.
Restriction sites
Specific Biotinylation via ViralBrick: The Biotinylation Acceptor Peptide
The purified BirA biotin ligase that was kindly provided from Avidity.com
References:

IMAC purification via Viral Brick: The Histidin Affinity Tag
The high binding affinity of Histidine towards metal is being exploited for the purification of proteins via the so called „Immobilized Metal Ion Affinity Chromatography“ (IMAC): Multiple histidine residues (most commonly: Six) are being fused to the end of the targeting protein. A cell extract containing the recombinant protein ist then applied to a collumn containing immobilized Ni2+-Ions. The His-tags covalently bind the Ni-Ions while other cellular proteins can be washed oft he collumn. The purified proteins can then be eluted with Imidazol, which displaces the histidine residues.(Smith et al. 1988), (Hoffmann & Roeder 1991)
Since the aim behind engineering therapeutic AAV vectors is a safe administration to human patients, it is important to consider a convenient way of purifying the virus particles. Contamination by cellular proteins could cause toxic side effects or a strong immune response. Koerber et al. have first inserted a His-tag into a surface-exposed loop at amino acid position 587 in the Cap protein and successfully purified recombinant virsuses using IMAC (Koerber et al. 2007). For our Virus Construction Kit, we provide the His-tag motif in the ViralBrick standard, allowing for an easy insertion into the 453 and/or 587 loop. If the modified capsid bearing a His-tag is being cotransfected with a wild type capsid for the production of mosaic viruses, IMAC helps to not only purify the produced viral particles but also to enrich particles which actually contain the modified proteins.
References:

Targetingpeptides via ViralBrick: The RGD Motif
References:

Coupling Antibodies to the Viral Surface via ViralBrick: The Z34C Motif
This engineered antibody binding domain of 34 amino acids was then inserted into capsids of different viral vectors amongst others also the AAV. In [Ried et al.; 2002] the Z34C domain was inserted at position 587 into the capsid of the AAV resulting in viral vector that can be targeted to different target cells without genetic engineering. This targeting approach was then improved in [Gigout et al.; 2005] by the creation of mosaic vectors that contain only ~25% of recombinant VP-Proteins what resulted in 4 to 5 orders of magnitude more infectiosity compared to all-mutant viruses.
References:


Risk assessment of the Adeno-associated Virus

Please consider special provisions of law in your country before using parts that contribute to the production of genetically modified viral vectors.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]