Difference between revisions of "Part:BBa J04450:Experience"
YuchenZhou (Talk | contribs) |
YuchenZhou (Talk | contribs) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 620: | Line 620: | ||
| − | ==Team NAU-CHINA 2023 | + | ==Team NAU-CHINA 2023 contribution: Effect of mRFP expression on the cell abundance measurements== |
====Purpose & Design==== | ====Purpose & Design==== | ||
| Line 664: | Line 664: | ||
<html> | <html> | ||
| − | <center><img src="https://static.igem.wiki/teams/4613/wiki/parts/parts/the-fluorescence-of-each-culture.png"with="1000" height="" width=" | + | <center><img src="https://static.igem.wiki/teams/4613/wiki/parts/parts/the-fluorescence-of-each-culture.png"with="1000" height="" width="750" height=""/></center> |
</html> | </html> | ||
| Line 675: | Line 675: | ||
<html> | <html> | ||
| − | <center><img src="https://static.igem.wiki/teams/4613/wiki/parts/parts/the-od-subabssub-and-deviation-analyses.png"with="1000" height="" width=" | + | <center><img src="https://static.igem.wiki/teams/4613/wiki/parts/parts/the-od-subabssub-and-deviation-analyses.png"with="1000" height="" width="750" height=""/></center> |
</html> | </html> | ||
Latest revision as of 11:26, 11 October 2023
This parent part was modified by Maddie Hunter at Davidson College to make a much stronger reporter called J100521. She flanked the part with two, 500 bp fragments of DNA from yeast and the RFP is much brighter. See the J100521 for details.
Team Aalto-Helsinki 2019 characterization: Effect of antibiotic concentration on fluorescence intensity
Aim
The aim was to characterize the RFP coding device (BBa_J04450) by measuring the effect of a range of different chloramphenicol concentrations on fluorescence intensity in Escherichia coli DH5a.
Method
For the experiment, a DH5a colony harboring the BBa_J04450 was used to inoculate 20 ml of LB medium supplemented with 25 ug/ml chloramphenicol. The culture was grown for 24 h at +37 C with 250 rpm shaking. The experiment was carried out on a 96-well plate with 90 ul of LB+cam and 90 ul of cell culture in each well. The following chloramphenicol concentrations were tested: 12,5 mM; 17,5 mM; 25 mM; 32,5 mM; 62,5 mM; 87,5 mM. Concentrations represent final concentrations in each well after addition of cell culture.
Measurement was carried out with Synergy H1 microplate reader (Biotek). Excitation read was set to 556 nm and emission to 586 nm. OD600 was measured from each well. Optics were read from the bottom of each well. Temperature was set at 37 C (+ preheat) and the plate was under continuous shaking with 230 rpm. Fluorescence and absorbance readings were measured every 10 min over 8 h. Mean fluorescence and OD600 values were calculated from three replicates at each time point. Fluorescence values were normalized with OD600 values so that differences in cell growth would be considered. As a reference for this method, we used a protocol kindly provided by Christopher Jonkergouw from Aalto University. The protocol can be found in our WIKI page under Protocols (18. Fluorescence expression in Microtitre).
Results
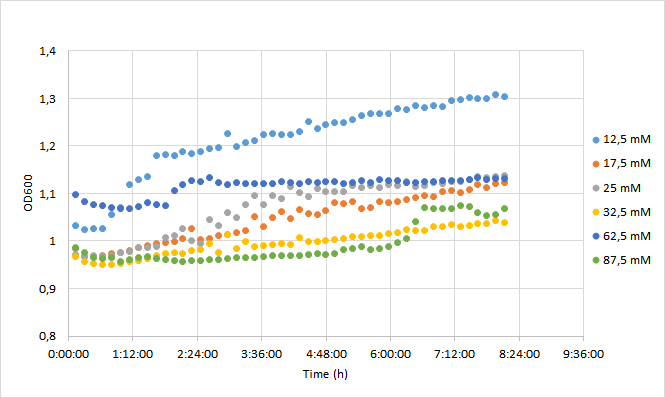
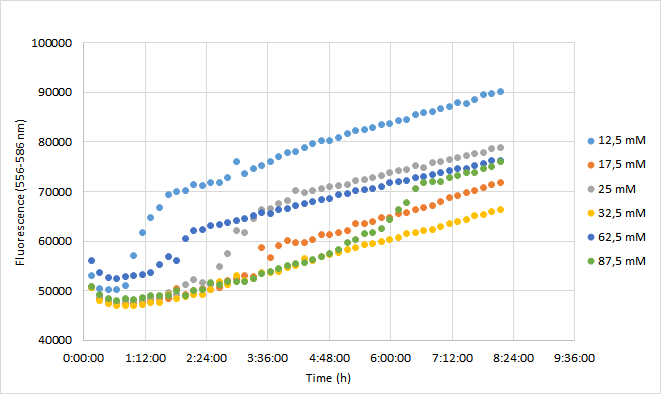
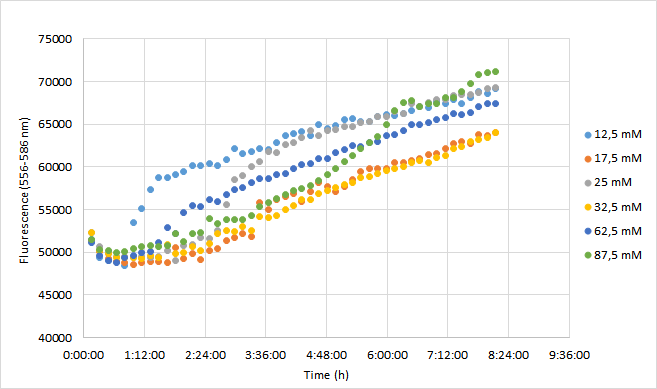
OD600 values for each tested antibiotic concentration are presented in Figure 1. Absolute fluorescence values are presented in Figure 2, and normalized fluorescence values in Figure 3.
Initial OD600 levels were between 0.1-1. Cell density of cultures supplemented with lowest antibiotic concentration (12,5) increased from 1.0 to 1.3 during 8 h incubation. Cell density of cultures with higher antibiotic concentrations increased slightly less, up to 1.1 approximately.
Fluorescence values were highest in cell cultures supplemented with lowest antibiotic concentrations (12,5 mM), following a similar pattern to cell density. Second highest fluorescence values were observed with cells supplemented with 25 mM, followed by 62,5 mM concentrations.
Normalized fluorescence values increased in all chloramphenicol concentrations we tested. Fastest increase was observed with the lowest antibiotic concentration. After 8 hours of incubation, highest antibiotic concentration showed the highest fluorescence readings. This is probably because cell density in 87,5 mM cultures increased after 6,5 h incubation.
In conclusion
It was shown that different chloramphenicol concentrations have an effect on fluorescence intensity. Lower chloramphenicol concentration (12,5 mM) which is typically applied could maintain RFP expression in DH5 for 8 h. It was also shown that cells were able to grow and express RFP with chloramphenicol concentration of 87,5, which is 3,5 times higher than the recommended concentration (25 mM). Cells supplemented with 17,5 and 32,5 mM concentrations had lowest expression of RFP of the range of concentrations studied here.
Team Lund 2019 charecterization: investigation of the correlation between the different strains and how the varying amounts of salts and sugars in the media stimulate cell growth and protein expression.
Aim
The aim of this characterization was to investigate the correlation between the different strains and how the varying amounts of salts and sugars in the media stimulate cell growth and protein expression. RFP was incorporated in the construct to compare the protein expression in various conditions. Three different E.coli strains were tested in three different complex media. The strains that were tested: E. coli TG1 E. coli BL21-DE3 E. coli Nissle 1917 (EcN) While the prepared media were: LB Media TB Media Enriched Complex Media The recipe for the three types of media can be found in our protocols.
Method
The plasmid from the registry was successfully transformed using heat shock to each of the mentioned strains. Three 1 L Erlenmeyer flasks containing 250mL of each media were prepared (9 flasks in total). Cultivation was performed at a temperature of 37 °C and 175 rotations per minute (rpm) , during which the growth was monitored spectrophotometrically. The protein expression could be detected optically from the color formation as well as with the use of Agilent Technologies Cary Eclipse Fluorescence Spectrophotometer at excitation wavelength of 532 nm and emission wavelength of 605 nm. For this experiment, a wavelength of 660 nm was used to measure optical cell density (OD) following the suggestion from iGEM headquarters.
The calibration curves for Rhodamine B (dye equivalent of RFP, obtained from Thermo Fisher Scientific - D1841) and for microbeads (equivalent of cells, provided by iGEM headquarters) were used to create a conversion factor for auxiliary fluorescence unit and Abs 660 nm. Two excitation wavelengths have been used 532 nm (recommended for RFP) and 570 nm (recommended for Rhodamine B). The protocol used to make the standard curves was the iGEM protocol “iGEM 2019 Plate Reader Fluorescence Calibration” for RFP.
Results
Standard curve
The emissions for these two excitation wavelengths can be seen in Figure 1 below.
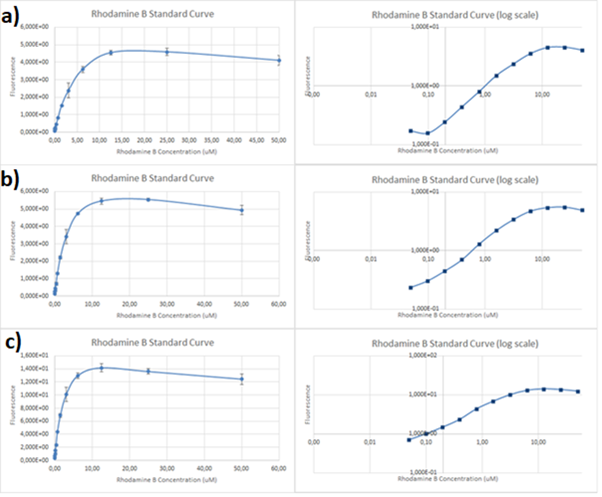
Figure.1 The emission spectra for various concentrations of Rhodamine B (0 to 50μM): a) excitation at 532nm; b) excitation at 570nm.
One can notice that at excitation wavelength of 532 nm, a clear emission peak can be obtained approximately at 590 nm with the increase in signal intensity for each Rhodamine B concentration. While at excitation wavelength of 570 nm there are no clear peaks but the signal increases with the concentration of the fluorescent dye. Therefore, several standard curves have been plotted. The 532 nm/605 nm (ex./em.) was the one that was used for RFP, the 605 nm was a result of fluorescence shift due to presence of RFP in the cells. The 532 nm/590 nm was the setting to check the theoretical emission Rhodamine B at 590 nm using the same excitation as RFP. The last set of wavelengths 570 nm/590 nm was recommended by the manufacturer of Rhodamine B. The results are presented in Figure 2 below.
Figure 2. Rhodamine B standard curves showing mean fluorescence of four replicates of different concentrations of Rhodamine B (0 µM to 50 µM): a) excitation: 532nm, emission: 605nm; b) excitation: 532nm, emission: 590;c) excitation: 570, emission: 590
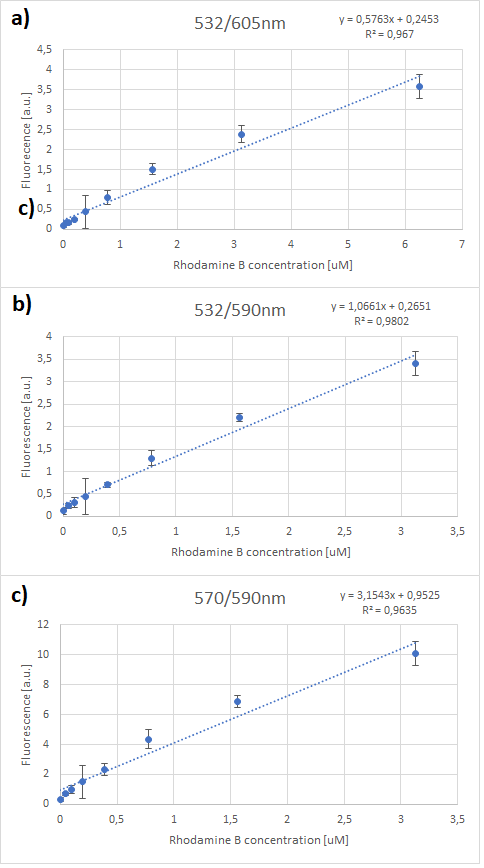
It can be clearly seen that in all cases there was an oversaturation of detector at concentrations of 12.5 μM and above. Therefore, it was decided that these concentrations should not be used for creation of standard curve and have been excluded. In case of 532/605, the noise is disturbing the measurement at lower concentrations but both 532/590 and 570/590 do not seem to have the same issues.
Figure 3. Rhodamine B standard curves showing mean fluorescence of four replicates of different concentrations of Rhodamine B (0 µM to 6.25 µM – the higher values were excluded due to oversaturation):a) excitation: 532 nm, emission: 605 nm; b) excitation: 532 nm, emission: 590 nm;c) excitation: 570 nm, emission: 590 nm
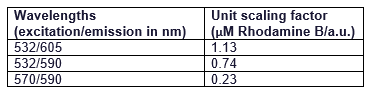
It can be seen that all the curves show similar linearity. The data from the curves was used to determine a unit scaling factor (μM Rhodamine B/ fluorescence a.u.) that can be found in Table 1, below.
Table 1. The mean μM of Rhodamine B per fluorescence auxiliary unit (a.u.)
Since all the standard curves have similar linearity but the scaling factors are significantly different, it was decided to choose the manufacturers recommendation and use the date from 570nm excitation and 590nm emission, that is 0.23 μM Rhodamine B/a.u.. It was hence used to convert the fluorescence auxiliary units in the characterisation experiment.
The calibration curve for the particles can be seen in Figure 4. below.
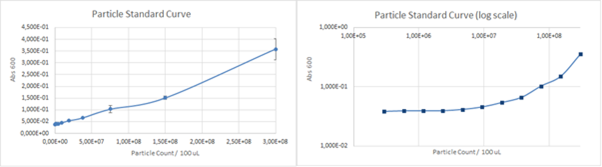 Figure 4. The calibration curve for absorbance of various concentrations silica particles at 660 nm.
Figure 4. The calibration curve for absorbance of various concentrations silica particles at 660 nm.
The calculated unit conversion factor was 1,23E+09 particles/Abs660 and it was used to convert units in characterization experiments.
The RFP/Rhodamine B Fluorescence Standard Spreadsheets found here for all excitation/emission can be found here:
Characterization results
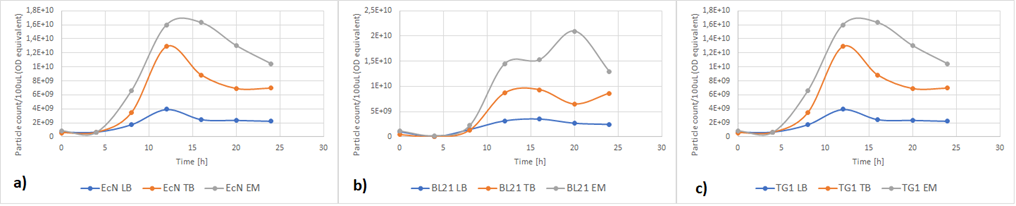
Figure 5a below shows the growth of EcN in different media in a fraction of time. Enriched Complex Media appears to be the most suitable for the growth of EcN. The lag phase is shorter compared to the other two media, while the logarithmic phase is longer. In TB Media the lag phase is extended, and the growth is not as high as before but still exhibits a clear logarithmic phase. In sharp contrast to that, the lag phase in LB Media is almost double and the exponential phase is quite brief. These results are expected since the media most enriched with salts and sugars are the media with the highest growth rate. The same applies to all strains (Figure 5b and Figure 5c).
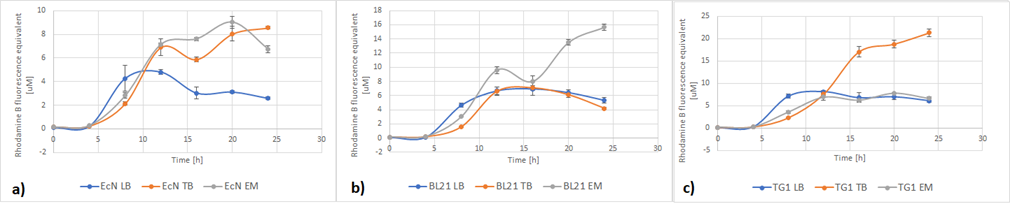
Figure 5. The growth of various E. coli strains expressing RFP in different media: a) EcN in LB (blue), TB (orange), EM (grey). b) E. coli BL21-DE3 in LB (blue), TB (orange), EM (grey) c) E. coli TG1 in LB (blue), TB (orange), EM (grey). The OD was converted to equivalent particle count/100μL.
Figure 6 below shows the expression of RFP in different media. In EcN (Figure 6a), the expression of RFP for TB and Enriched Media are comparable, whereas the RFP production in LB is significantly lower. For E. coli BL21-DE3 (Figure 6b), however, the expression levels in TB and LB Media are similar and in Enriched media the RFP production was considerably higher. Finally, in E. coli TG1 (Figure 6c) contrary to the other two strains the Enriched Media gives the lowest RFP levels. The expression in LB media is only slightly higher than in Enriched media. The protein expression in TB Media, however, is notably higher than the other two media, and in comparison to the other strains in the same media. In all three strains, the production of the protein was initiated in LB Media but it could not reach as high values as in the more fortified media, which was expected.
Figure 6. The expression of RFP in various E. coli strains in various cultivation media: a) EcN in LB (blue), TB (orange), EM (grey). b) E. coli BL21-DE3 EcN in LB (blue), TB (orange), EM (grey) c) E. coli TG1 EcN in LB (blue), TB (orange), EM (grey). The OD was converted to equivalent particle count/100μL.
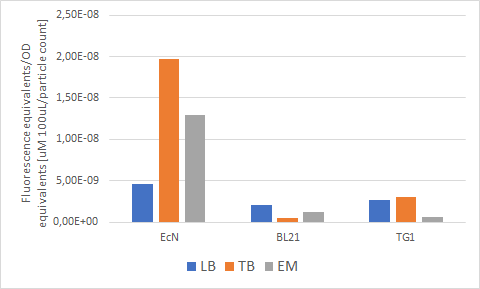
Figure 7 below shows the expression of RFP (converted to Rhodamine B equivalents) per OD (converted to particle/100uL). When it comes to growth, all strains prefered Enriched media. However, it can be noted that each strain has a different preference regarding the RFP expression. EcN produces the most RFP per cell in TB media, BL21 in LB media while in TG1 the expression is favored in both LB and TB media.
Figure 7. The production of RFP per particle count (OD equivalent) for each strain in various media at the end of cultivation: LB (blue), TB (orange), EM (grey).
Figure 8 displays growth of the three strains of E.coli during the characterization of RFP. Overall, the darkest red color was from cells grown in TB media. This was where the protein expression was higher. The other two media, complex Enriched media and LB media both gave a quite pale pink color and low protein expression, with one exception of the BL21-DE3 cells which gave a dark red color and high protein expression. For BL21-DE3 cells, the complex enriched media showed the darkest color. For TG1 cells the TB media gave the darkest color. For EcN cells the TB media also gave the darkest color. These correspond to the results presented in Figure 7.
Figure 8. Growth of three strains of E.coli cells transformed with RFP plasmid BBa_J04450. The cells were grown in different media. The three flasks on the left were grown in TB media, and the strains were from left to right; BL21-DE3, TG1, EcN. The three flasks in the middle were grown in complex Enriched media, and the strains were from left to right; BL21-DE3, TG1, EcN. The three flasks on the right were grown in LB media, and the strains were from left to right; BL21-DE3, TG1, EcN.
Team Grenoble-Alpes 2019: characterization of the effect of cAMP on the promoter’s leakages and protein expression in Escherichia coli
Purpose
The goal here is to characterize the effect of cAMP on the promoter’s leakages.
To do that we used the cAMP depleted Escherichia coli strain BTH101. In this strain the cya gene has been deleted (cya-) preventing the expression of endogenous adenylate cyclase and so the production of cAMP.
EXPERIMENT
Here we transformed BBa_J04450 in:
DH5α which is a standard strain with endogenous adenylate cyclase
activity.
BTH101 which is a strain without endogenous adenylate cyclase activity
and so without production of cAMP. BTH101 strain is streptomycin
resistant.
BTH101 + adenylate cyclase (BTH101-Zip) to generate cAMP in the
strain.
To restore the adenylate cyclase activity we used two plasmids: one
containing pUT18-LeucineZipper (pUT18-LZ) and the other containing
pKT25-LeucineZipper (pKT25-LZ). The Leucine Zipper’s (LZ) will
homodimerize and will bring T18 and T25 closer which will restore the
adenylate cyclase activity thus allowing the production of cAMP by
BTH101. Both T18 and T25 are under the control of an IPTG inducible
promoter (lactose promoter) to create an auto amplifier system.
BBa_J04450 was cloned in pKT25-LeucineZipper, and the bacteria was co-
transformed with both plasmid T25-LeucineZipper + BBa_J04450 and
T18-LeucineZipper.

DH5α, BTH101 (without cAMP) and BTH101-Zip (with cAMP) were tested as follow:
A range of IPTG concentrations and several times of induction were tested.
Relative Fluorescence Units (RFU) where recorded with an excitation
wavelength of 561nm and emission wavelength of 588nm.
The assay was performed in triplicate in a 96 wells NUNC plate.

RESULTS
Transformation

It is already apparent that without IPTG there is a visible difference between
strains that can produce cAMP (DH5α and BTH101-Zip) when compared with the BTH101 strain that do not produce any cAMP.
The DH5α red colonies reveal a substantial RFP expression as the result of the
leakage of the promoter.
BTH101 has white colonies indicating that no visible RFP was produced. This data
suggest that the promoter does not leak in that condition.
BTH101-Zip presents same red colonies than the DH5α strain.
Measurements

In a strain producing endogenic cAMP the lactose promoter has a non-
negligible leak which triggers RFP expression even without any IPTG induction.
The amount of RFP increases over time and IPTG concentration. Furthermore,
the longer the induction time the greater the effect of IPTG seems to be.

Strain without endogenous cAMP synthesis has a basal expression lower than the strain that can produce endogenic cAMP.
Neither time nor IPTG have any effect on the RFP expression probably because no lactose promoter activation occurs.
The data suggest that cAMP is essential for the transcription of genes located downstream the lac promoter unlike IPTG.

When the adenylate cyclase activity is restored, cAMP is produced as shown in the BTH101-Zip strain. The level of expression of RFP is comparable to that obtained in DH5α strain indicating that cAMP is an essential factor for transcription under the control of the lactose promoter.
The endogenic and uncontrolled production of cAMP is the cause of the leakage. As a consequence, making the cAMP production dependent on the presence of IPTG the leakage is greatly reduce in this strain.
Because T18-Zip and T25-Zip are under an IPTG inducible promoter they express very low level of RFP in absence of IPTG (very few T18 and T25 are expressed and so very few cAMP are produced).
Conclusion

RFP expression by BTH101 with or without cAMP shows that without cAMP, the lactose promoter has almost no leakage suggesting that the leakage of the lactose promoter is due to cAMP in absence of IPTG. When the cAMP production is restored the promoter is activated and the protein is expressed.
It is also interesting to note that with the BT101-Zip there are very little protein expression if no IPTG is added.

Usage and Biology
Using cAMP depleted strains and T18-leucineZipper/T25-leucineZipper under the control of a lactose promoter is a good alternative to produce inducible proteins without leakage
Team Grenoble-Alpes 2018: Delay before fluorescence apparition and leaky expression after transformation in three Escherichia coli strains
The main goal of this characterisation was to find out which E. Coli strain was the most effective among three different strains (TOP10, DH5α and BL21), to obtain a rapid and high red fluorescence with pSB1C3-BBa_J04450.
We also studied the influence of adding 0.3mM of IPTG on the RFP expression in these three strains to see if the leak was different between host strains.
Indeed, former iGEM teams had described a leaky expression of fluorescence with this biobrick, which means there was no need to induce expression with iPTG to obtain a high rate of fluorescence.
As we raised the question in the context of our project, we thought it might be useful to know which E. Coli host strain is the better to use depending on the conditions you want. For instance, you might want to strictly control RFP expression and so you will need to reduce the leaky expression at most. On the contrary, you may want to have the highest leak as possible to avoid using IPTG, a need our team encountered for the using of our automated system.
</p>
We wanted to test three E. Coli strains usually used in laboratories : DH5α, TOP10 and BL21, to see, in the context of our system, which one would be the host with the fastest and highest production of RFP.
We used a bacterial stock of our own, coming from commercial bacteria cultured and made competent using our protocol <a href="http://2018.igem.org/Team:Grenoble-Alpes/protocols">(see competency protocol here)</a> a few weeks before the experiment and conserved at -80°C.
We made an heat-shock transformation <a href="http://2018.igem.org/Team:Grenoble-Alpes/protocols">(see transformation protocol here)</a> of 23ng pSB1C3-BBa_J04450 in 25μL of each bacterial strains. We then cultured transformed bacteria in 250μL LB broth medium for one hour until they expressed chloramphenicol resistance. Then, 750μL of LB broth was added with chloramphenicol and incubated for one hour. After centrifuging the bacteria for 10 minutes at 5000 rpm, we replaced the old medium by resuspending the bacterial pellet. We also split the samples in half, and put each new samples in 30mL LB broth with chloramphenicol, completed or not with 0.3mM of IPTG.
We then measured red fluorescence (620nm) and O.D.600nm every hour for 27 hours.
NB: We had to do O.D.600nm manually as the TriStar microplate reader wasn’t able to do such measurements. Due to the experiment duration and the limited access to the laboratory, we were not able to make O.D.600nm measurements between 9 hours and 18 hours in the experiment. For the night, we thus launched automatic measurements for fluorescence, but we do not have any O.D.600nm linked to these measurements.
The results are represented in the following figures.
The data presented here start 17 hours or 18 hours after transformation because we didn’t observed any fluorescence until this point. This might be due to the huge culture volume in which we put bacteria (30mL). A huge volumed allowed us to make a long experiment with lots of measurements, but it also diluted a lot the bacteria and their fluorescence.

Figure 1
The first figure shows the fluorescence obtained for each sample, without taking into account the number of bacteria in the sample. These results were useful as a part of our project, as we wanted the highest fluorescence rate with the lowest time of apparition after transformation to improve the sensibility and speed of our detection system, regardless of the corresponding amount of bacteria.
The figure shows that fluorescence obtained with TOP10 E. Coli was a lot higher than the ones obtained with either BL21 or DH5α. 620nm measured fluorescence for TOP10 was twice as high as the one obtained with BL21 and more than 7 times as high as the one for DH5α. We also observed fluorescence a lot sooner with the TOP10 bacteria : 18 hours after transformation versus 23-24 hours with the other strains.
As for the DH5α and BL21 comparison, we can see that DH5α had a much lower fluorescence rate, whereas BL21 gave pretty good results when induced with ITPG.

Figure 2
The second figure represents the fluorescence rate rectified with O.D.600nm measurements. This allows to compare in a more quantitative way the fluorescence obtained, as if for each point, we had the same number of bacteria. It must be noticed that the differences in O.D.600nm were not clearly significative between the samples, which means that we can still compare them as they probably were in the same growing phase.
On this figure, we can see that there is no significant difference between the experiment with fluorescence only, so we can make the same observations as in figure 1.

Figure 3
The third and last figure represents the same results as in figure 1, but we made close-ups to better study each strain and the differences between induced and non induced promoter.
For the DH5α, we can see that there is a higher fluorescence rate for 0.3mM IPTG than without induction. However, we can still observe fluorescence without induction, which corresponds to the promoter leak : it represents almost half of fluorescence obtained with IPTG.
For the TOP10, we can see that there is almost no difference between the patterns obtained with or without induction. This means that the promoter leak is so important that IPTG induction is not significantly useful at all : the biobrick may be transformed and the RFP expressed in this host without using IPTG.
For BL21, the difference between induced and non-induced expression is clearly apparent : there is almost no fluorescence without IPTG, whereas expression with induction began to increase exponentially from 23 hours after transformation. This strain appears to be the most effective if you need to reduce the promoter leak as much as possible.
Results showed the differences between E. Coli host strains, which allow variable RFP expression rates, different delays before fluorescence apparition, and also particular responses to induction. The results are summarized in the following table :
| E. Coli strain | DH5α | TOP10 | BL21 |
|---|---|---|---|
| Delay before fluorescence observation | ±22 hours | ±18 hours | ±22 hours |
| Fluorescence rate with IPTG | Low | Medium | High |
| Promoter leak | Medium | Extremely high | Extremely low |
The delay before fluorescence must be analyzed with caution, as it might have been affected by the huge culture volume compared to the start amount of bacteria.
The fluorescence rate and promoter leak must be further studied in these three strains with higher concentrations of IPTG to see if we would obtain different results.
Team Kingsborough NY 2017
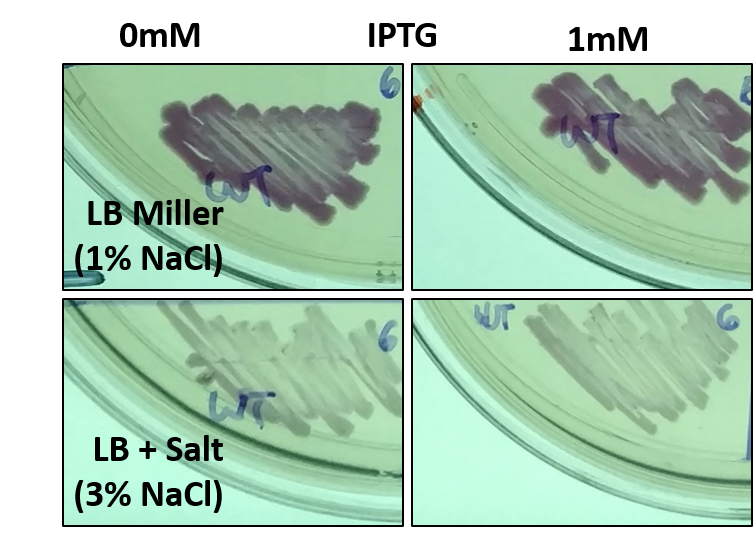
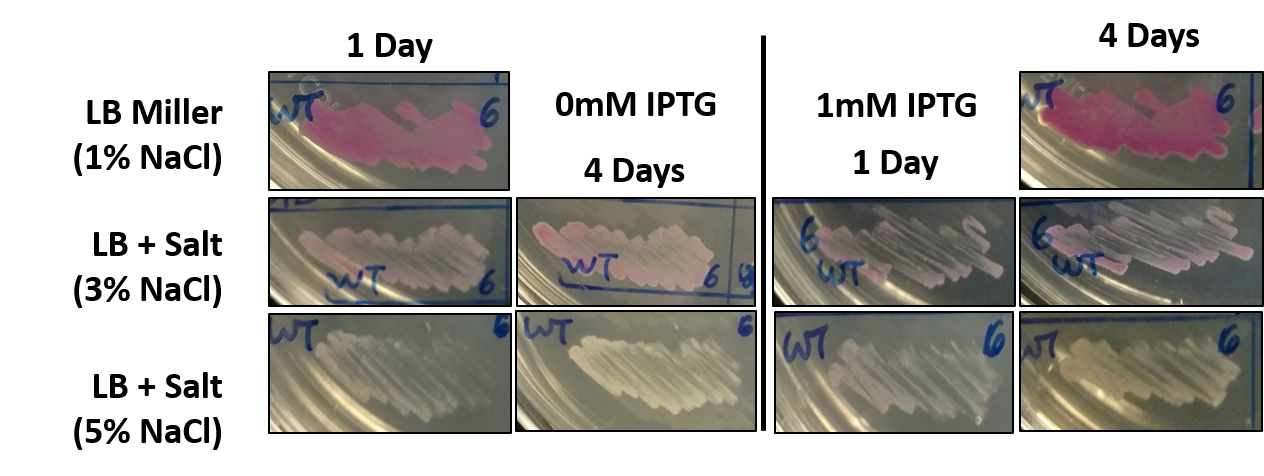
Part of our project involved assessing the risk of escape posed by a genetically modified organism (GMO), especially one being employed in a wastewater treatment facility. Towards this end, we grew E. coli harboring this part (BBa_J04450) as an example GMO in different conditions, some of which mimic the salinity of seawater (typically 3-5% NaCl w/v). Surprisingly, we noticed that while the bacteria could grow on the higher salt media, the expression of RFP was decreased. This was unexpected, as the promoter in this part that drives RFP expression is not characterized as being salt responsive. We further confirmed this result by directly streaking E. coli with this part on different media.
Fig 1: E. coli with BBa_J04450 streaked onto LB + Chloramphenicol plates supplemented with 1mM IPTG and/or 3% NaCl. Since LB Miller already contains 1% NaCl w/v, when preparing 500mL of LB Agar another 10g of NaCl was added to the mixture before sterilization. The bacteria was streaked using a sterile toothpick and a parent colony grown under normal conditions. The plates shown are representative of multiple experiments, and display bacteria after 1 day of growth at 37C.
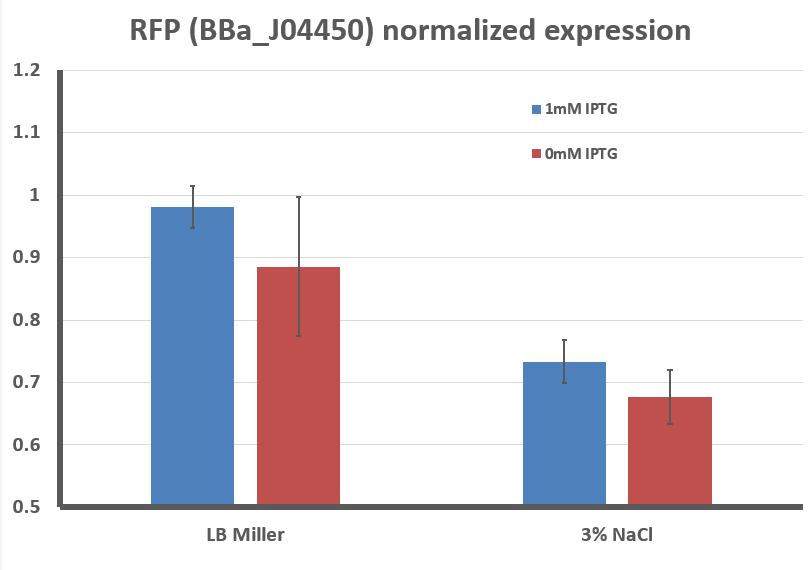
Fig 2: Quantified levels of RFP expression for E. coli with BBa_J04450 grown for 1 day on LB + Chloramphenicol plates supplemented with 1mM IPTG and/or 3% NaCl. Images of the plates were taken and the Grayscale, Red, Green, and Blue levels in each image were determined using ImageJ. RFP expression per cell is approximated by the amount of Red color over a set of pixels divided by the level of Green color. This difference is normalized to the maximum value obtained in all experiments. Graph shown is an average of at least 3 separate experiments, error bars represent standard deviation. Differences between LB Miller and 3% NaCl media were shown to be statistically significant using Student's T-Test (p < 0.05).
Bacteria harboring this part not only display decreased RFP expression in high salt, but also appear to grow slower. This was true for media containing 3% NaCl, but even more severe for media with 5% NaCl. However, allowing additional time for growth at high salt does not allow the bacteria to recover the levels of RFP expression seen after 1 day of growth in normal media.
Fig 3: E. coli with BBa_J04450 grown for 1 day on LB + Chloramphenicol plates supplemented with 1mM IPTG and/or 3% to 5% NaCl. Plates were allowed to grow overnight at 37C for either 1 or 4 days, and photographed against a dark background.
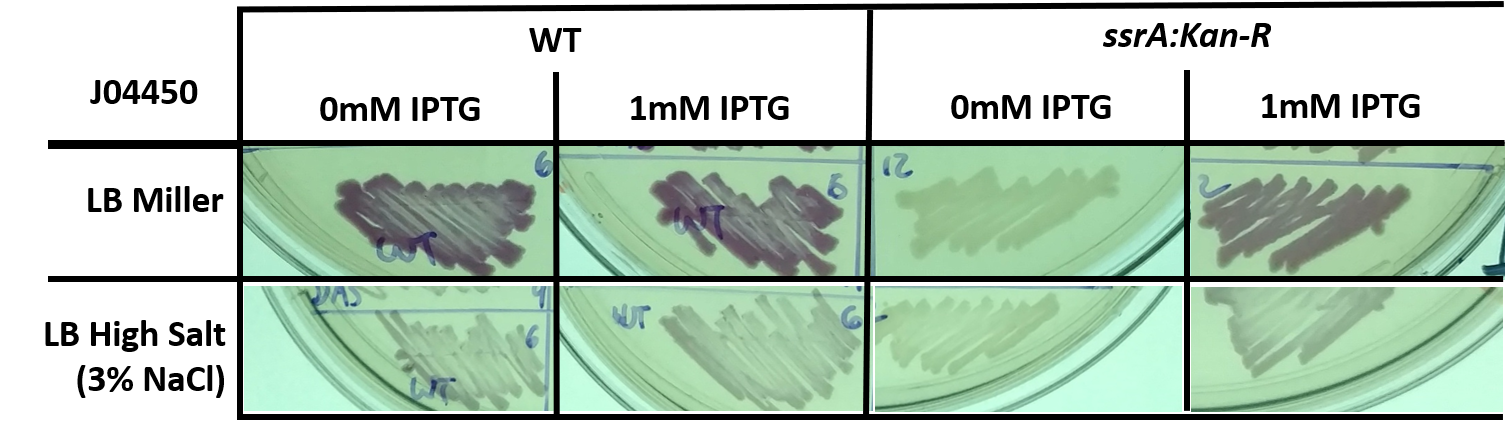
In addition to a response to high salt media, we also wished to observe how RFP expression changes in different genetic backgrounds - namely, in E. coli with the ssrA::Kan-R insertion, which lack tmRNA and trans-translation ribosome rescue. We used part J04450 as a control for experiments with parts K1399010 through K1399013 (ssrA tagged RFP).
Fig 4: E. coli, either wild-type (WT) or trans-translation deficient (ssrA::Kan-R) with BBa_J04450 streaked onto LB + Chloramphenicol plates supplemented with 1mM IPTG and/or 3% NaCl. The bacteria was streaked using a sterile toothpick and a parent colony grown under normal conditions. The plates shown are representative of multiple experiments, and display bacteria after 1 day of growth at 37C.
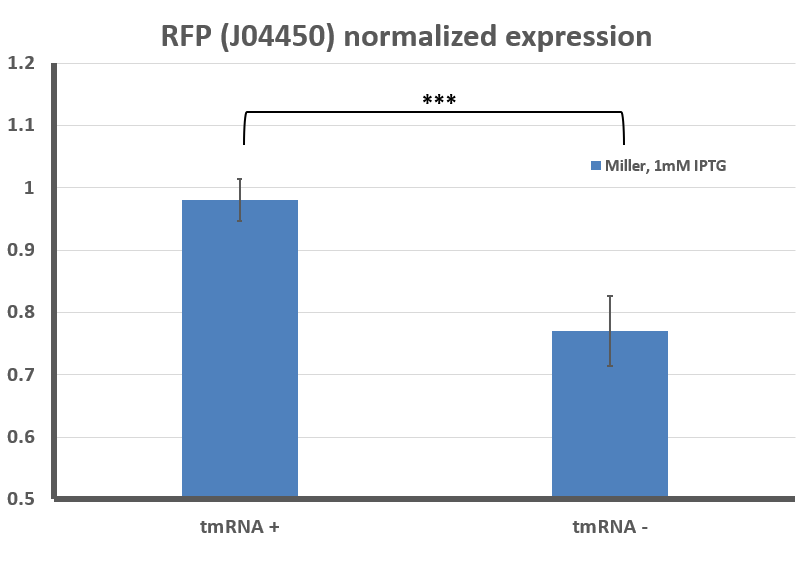
Fig 5: Quantified levels of RFP expression for E. coli, either wild-type (WT) or trans-translation deficient (ssrA::Kan-R) with BBa_J04450 streaked onto LB + Chloramphenicol plates, and/or supplemented with 1mM IPTG. Data analysis carried out the using the same method described in Fig 2. Graph shown is an average of at least 3 separate experiments, error bars represent standard deviation. Differences between LB Miller and 3% NaCl media were shown to be statistically significant using Student's T-Test (p < 0.05).
The tighter regulation of gene expression (no leaky expression at 0mM IPTG) and overall lower steady state level of RFP in the ssrA:Kan-R is not a complete surprise - others have described how LacI is a substrate for the tmRNA tagging system, and that induction of LacI responsive promoters is usually lower in a background that does not feature tmRNA-mediated ribosome rescue. However, previous studies have featured only a difference in the dynamics of gene expression, and did not observe a difference in steady state levels, as we do here (Abo T, 2000)
Team TecCEM 2017
The objective of this characterization was to evaluate the moment of maximum expression of the protein and its behavior with the induction with IPTG taking into account the previous characterizations that RFP is optimally expressed with 20 mM IPTG. The complete protocol can be consulted in: http://2017.igem.org/Team:TecCEM/Improve

Figure 1. Basic steps for RFP characterization followed by team TecCEM
We measured biomass and fluorescence for 8 hours and made the corresponding curves.

Figure 2. Graph of the biomass growth of the induced and non induced cultures of DH5α + BBa_J04450

Figure 3. Statistical analysis of the measures of RFP obtained through 8 hours of induction.
We performed also two different 10% SDS PAGE for visualizing the 26 kDa band corresponding to the Red Fluorescent Protein. Each gel was loaded with the non-induced sample and its induced counterpart. However, during the previous treatment, we did not incubate the samples at 100ºC in the water bath, while we did for the samples of the other gel. By not performing this step, the protein was not denaturalized and was seen under the transilluminator.


Figures 4 and 5. Comparison between RFP expression in DH5𝑎 induced and no induced with IPTG.
In these figures, we can also see that by each hour the expression of this protein and the fluorescence intensity increased over time until the eighth hour, where it is maximum. After the eighth hour, both biomass and fluorescence intensity start de decay, so any other team that wants to use this BioBrick to induce the expression of any interest protein in liquid medium will have the highest yield after eight hours.
Team Grenoble-Alpes 2017
The colonies of bacteria transformed with this plasmid are clearly red under natural light after about 18 hours. Smaller colonies are visibly red under UV. The RFP part does not contain a degradation tag and the RBS is strong. This part is commonly used, but can fail if the system contains LacI or CAP protein. (Informations from iGEM website, "Parts" section, see here http://2017.igem.org/Team:Grenoble-Alpes/Parts).
The promoter of mRFP1 gene in each plasmid is supposed to be inducible (with IPTG) but a strong leak of RFP production could be observed. (fig 1)

Figure 1: Observation of the leak of the promoter. A : Bacteria transformed or not with BBa_J04450 sequence, naked eye, white light. B. Bacteria without mRFP1 gene, excited at 546nm. C. Bacteria with mRFP1 gene, excited at 546nm. Red fluorescence is notable.
Photos of the fluorescence are taken thanks to SnapLab, the biodetection kit of iGEM Grenoble 2017 (fig 2).
 Figure 2: SnapLab, the detection kit made by Grenoble-Alpes iGEM Team
Figure 2: SnapLab, the detection kit made by Grenoble-Alpes iGEM Team
-> Does IPTG induction bring forward and/or increase RFP production ?
A difference of fluorescence was evaluated, either on the intensity either on the time of apparition of the fluorescence. The following experiment has been designed in this aim.



Results showed IPTG had an importance in RFP production. Indeed, we can see that IPTG allows a shorter rise of fluorescence. It could be interesting to repeat this experience with different IPTG concentrations, to see whether the delay of apparition of the red fluorescence would decrease with a higher amount of inducer.
Team Warwick 2016
This year, our team demonstrated that this part can be triple transformed into the same competent cell while retaining the functionality of all the plasmids. This was demonstrated using pSB1C3, pSB3T5, and pSB4A5.
pSB4A5 is a BioBrick standard vector with a pSC101 replication origin, whilst pSB3T5 has a p15A replication origin. As both of these origins rely on iterons as the negative copy number control, the competition between the plasmids may render them incompatible when in the same cell[1], potentially limiting triple transformation efficiency. However through our experiment we show that the replication origins do not interfere with each other.
To illustrate this, we transferred the triple transformed cells onto plates containing chloramphenicol, tetracycline, and ampicillin, as shown in Figure 1 below. From this plate, we inoculated a single colony into water and streaked this water onto one half of three different plates containing one each of chloramphenicol, tetracycline, and ampicillin. On the other half of the plates, we streaked a colony of top10 cells that had been grown on a streptomycin plate. The results can be seen below in figures 2 and 3. Figure 3 shows the four plates under UV light, clearly showing where colonies have grown. As the triple transformed cells have grown on every single plates and no colonies have grown from the untransformed cells, this shows that plasmid functionality is retained.

Figure 1: Visible light image of triple transformation plated on Chlor-Amp-Tet plate

Figure 2: Visible light image of triple transformation plated on Chlor, Amp, and Tet plates alongside untransformed Top10 cells

Figure 3: UV light image of triple transformation plated on Chlor, Amp, and Tet plates alongside untransformed Top10 clearly showing differences in growth
Team Warwick 2015
Our team considered using this part as part of a system of binding different coloured cells together in order to demonstrate specific cell placement. We characterised this part is order to determine the optimal amount of IPTG required for inducing the gene, and the copy number necessary to express the fluorescence brightly. The results for this can be seen below.
We cloned J04450 into three plasmid with varying copy numbers, in order from highest to lowest copy number they are: pSB1K3, pSB3K3, and pSB4K5. These plasmids were then transformed into electrocompetent MG1655 Z1 cells and grown overnight. THe next morning the cells were refreshed, and different concentrations of IPTG (0uM, 250uM, and 500uM) were added to induce them. For each of the three plasmids in each IPTG concentrations, three biological replicates were made, and when OD600 and RFP absorbance were measured, three technical replicates were made, for a total of 81 copies of the gene grown. The RFP absorbance and OD600 of these cells were measured over 20 hours. The OD600 over time was used to determine at what OD the cells were in steady state. This was then compared to the RFP measured at that time and graphed to show RFP expression per cell.

The graph shows that RFP expression was highest in the pSB1K3 and pSB4K5 plasmids, and that there was little difference in expression between the 250uM and 500uM concentration of IPTG. 0uM IPTG universally showed almost no expression. pSB1K3 should have the highest copy number and pSB4K5 should have the lowest copy number, so it's curious that they both expressed RFP very well. This could be due to a mutation in the pSB4K5 causing it to have a much higher copy number than usual. It is documented here (http://www.ncbi.nlm.nih.gov/pubmed/1283002) that a single point mutation can increase the copy number of a plasmid. The pSB4K5 plasmid we tested has been sent for sequencing in order to determine whether this is the case.
The raw data for this characterisation can be found here:
https://static.igem.org/mediawiki/2015/7/70/Warwick_J04450_raw_data.txt
https://static.igem.org/mediawiki/2015/b/bc/Warwick_J04450_in_pSB1K3_analysed_results.txt
https://static.igem.org/mediawiki/2015/8/8f/Warwick_J04450_in_pSB3K3_analysed_results.txt
https://static.igem.org/mediawiki/2015/5/54/Warwick_J04450_in_pSB4K5_analysed_results.txt
https://static.igem.org/mediawiki/2015/6/64/Warwick_J04450_RFP_OD_analysed_results.txt
Team NRP-UEA_Norwich 2014
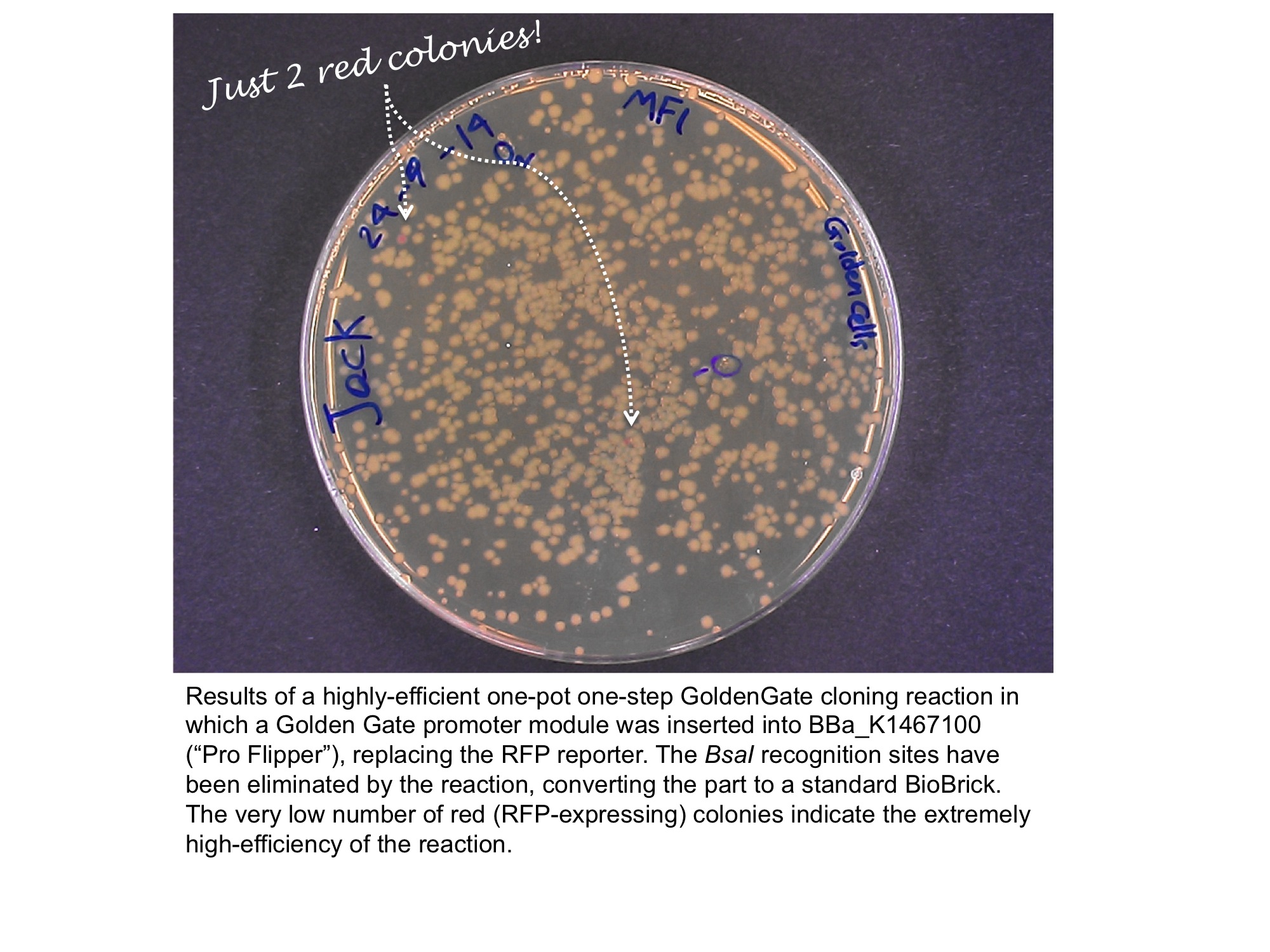
Our team has converted this part into a series of four "GoldenGate Flipper modules" that can be used to convert four types of standard part defined in the GoldenGate MoClo Assembly Standard into standard BioBricks. This was done by inserting an inverted pair of BsaI recognition sequences either side of the original RFP reporter part. The flipper reaction is a one-pot, one-step, digestion-ligation GoldenGate cloning reaction. When cloning is successful the colonies become white as the RFP sequence is replaced by the new part. After the reaction is complete, no part of the BsaI recognition sequence will remain between the BioBrick suffix and the part.
Four types of Golden Gate Parts can be converted. The four new parts differ by the identity of the 4 base-pair 5' overhangs produced by digestion with BsaI.
Bba_K1467100 Pro + 5U MoClo Flipper (GGAG-AATG)
Bba_K1467200 CDS1 MoClo Flipper (AATG-GCTT)
Bba_K1467300 3U + Ter MoClo Flipper (GCTT-CGCT)
Bba_K1467400 Transcriptional Unit MoClo Flipper (GGAG-CGCT)
The image below shows the sequences inserted (in orange) between the RFP and the BioBrick prefix and suffix that enable the flipper to accept MoClo Pro+5U parts (which begin GGAG and end AATG) in a one-step digestion-ligation Golden Gate cloning reaction.
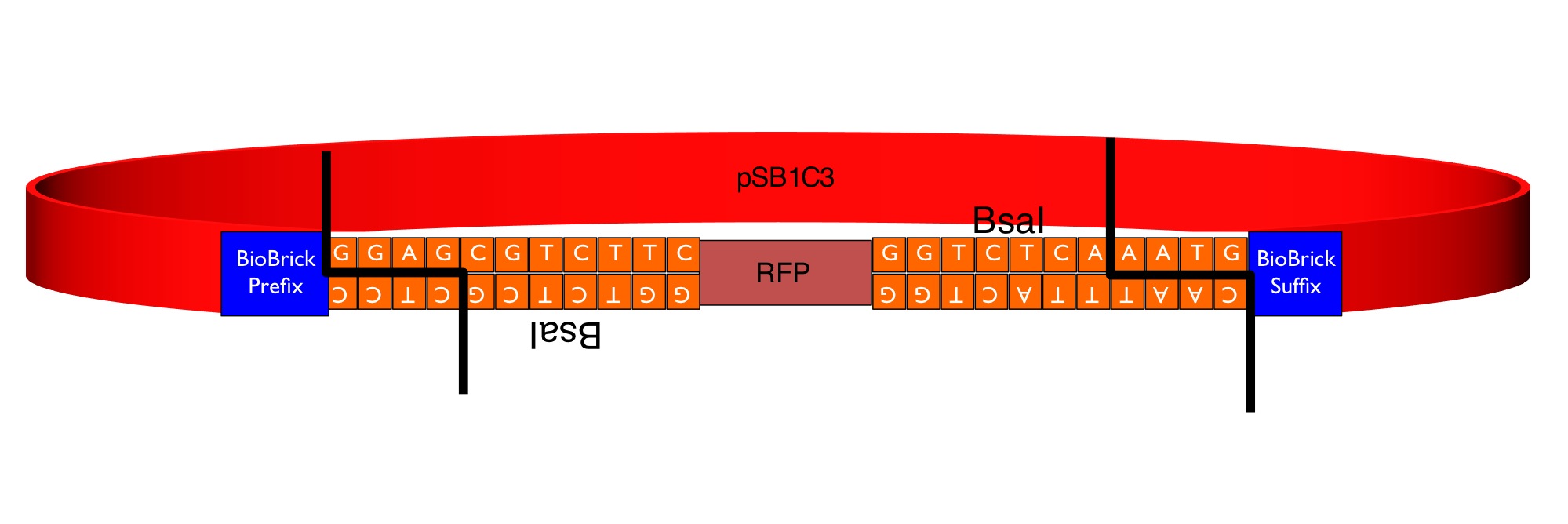
Below is an example of a Golden Gate one-step, one-pot cloning reaction with the improved BioBrick BBa_K1467100, where the restriction enzyme, ligase and (intact, uncut) donating and recipient plasmids were all incubated together in a single reaction before transformation into competent E.coli cells. Cloning was very successful as the vast majority of the colonies are white as the RFP sequence was replaced by the new part.
Team OUC-China 2014
This year our team use this part as a reporter gene link with BBa_k1439000. In order to mark our function plasmid. we find some problem about this part. The result shows Part BBa_J04450 didn’t work well as the reporter gene of the plasmid. The expression quantity of RPF is low or even next to zero under the condition of no resistance screening.

Figure1.
Figure1. Top10 strain containing BBa_J04450(pSB1C3).Culture medium contains no chloramphenicol to the left, to the right contains chloramphenicol. Under the same condition,37℃ 12h,RFP didn’t express in the culture medium without chloramphenicol.
Team NRP-UEA_Norwich 2013
Our team improved this part by adding an NdeI restriction site in front of the promoter region, upstream of the RFP gene. This means a restriction digest can be performed and the promoter or RFP gene exchanged to a allow further cloning. Please see part BBa_K1041000
Our aim was to evaluate RFP expression quantity of E. coli. In order to obtain this information, we agreed that the measurement of fluorescence intensity in certain OD values is the best approach. However, fluorescence quenching was a setback which we must overcome for accurate and reliable results. Because, as a result of quenching, fluorescence intensity counts do not give the actual amount of fluorescence expected from a certain OD value. Therefore we employed a curve fitting method suggested by Zhang et. al (2010) which is claimed to take the quenching problem into consideration

Where If is the fluorescence intensity; OD is the cell density; y0 is the offset; A1 is the amplitude; and t1 is the decay constant.
We chose BBa_J04450 for this experiment. And our negative control was again BBa_B0034.
There were nine bacterial concentrations prepared for one to five hours of IPTG induction. (All concentrations were prepared as triplicates.) Then a complete graph of all the induction durations was plotted with the help of the function shown above:

As seen in the graph, fluorescence behaves exactly as expected and does not increase linearly with the increased bacterial concentration. However, IPTG induction effect is clear on fluorescence intensity. (Error bars were too small to be visible.)

Finally, the variables A1 and t1 of the fittings of each induction duration were used to obtain a mean fluorescence intensity (MFI) value:



By conducting this experiment, we were able to characterize RFP expression pattern of the part BBa_J04450 in the host Top Ten. Theoretical mean fluorescence intensity reflects the fluorescence quantum efficiency of the fluorophore RFP, and was found to be unrelated to the cell concentration. Since it is obvious from the results we have obtained that change in the OD does not correlate linearly with the change in the fluorescence intensity. Therefore this model proposes a solution to the unpredictability of the fluorescence amount coming from a certain bacterial population.
The characterization was performed by METU-TURKEY 2010 IGEM TEAM.
Applications of BBa_J04450
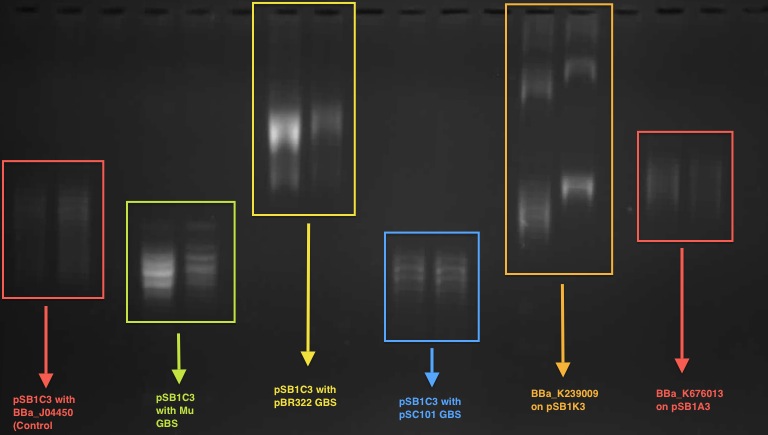
According to our result in the 1D chloroquine gel electrophoresis (at 2.5 μg/mL), the plasmid with the BBa_J04450 (on pSB1C3 backbone) migrated further compared to a plasmid with a known GBS (from pBR322 plasmid - BBa_K676012) on pSB1C£. At the chloroquine concentration which we used, the more negatively supercoiled plasmid will migrate further while the more relaxed molecules will travel slower through the gel.
So this indicates that the device BBa J04450 might have a potential gyrase binding site which facilitates the introduction of negative supercoils into plasmid compared to pBR322 GBS.
This characterisation was carried out by the UCL iGEM team 2011.
Tianjin iGEM 2012
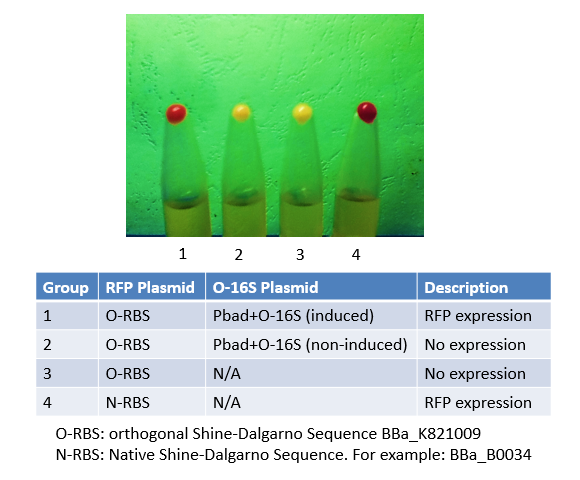
Originally, we used this BioBrick to express RFP in Group 4. We mutated the Shine-Dalgarno sequence of this BioBrick to make an orthogonal counterpart, BBa_K821001. As shown in the image, No. 4 is the product of this BioBrick, and No.1 is the product of its orthogonal counterpart. The results justified both this BioBrick and our BBa_K821001 worked very well, and expressed considerable amount of protein.
Glasgow iGEM 2011
It was found that the Red fluorescence of this protein was not consistently apparent when expressed in biofilm - although some results were observed. Numerous attempts to transform this construct into E. coli Nissle 1917 cells in a biofilm forming assay were unsuccessful, however, the RPF expressed readily into E. coli Nissle 1917 cells when grown on an agar plate.
The antibiotic resistance was still carried across when the culture was inoculated into the biofilm assay indicating that the plasmid was still present. The cultures were shaken at room temperature overnight.
It was thought that the lack of fluorescence was caused by the anoxic conditions generated by the biofilm assay, which may have resulted in poor folding of the chromophore. These results show that RFP is not the most efficient reporter molecule for biofilm, we would recommend something like iLOV, a new biobrick submitted by the Glasgow iGEM team this year [1].
Glasgow iGEM team 2011
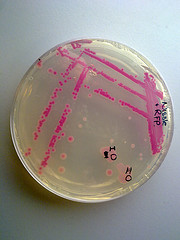
Haynes Lab, 2012
I used BBa_J04450 to demonstrate the expression of RFP in E. coli for a biomedical engineering course at Arizona State University.
- Cells: NEB10B
- Medium: LB Lennox agar, 100 ug/mL ampicillin
- The yellow/green area contains bacteria expressing GFP from BBa_I13522
User Reviews
UNIQ38be9daa3368cba9-partinfo-00000007-QINU
|
•••••
Antiquity |
This review comes from the old result system and indicates that this part worked in some test. |
|
•••••
|
We've used this part as an alternative to the suicide part p1010 (ccdB) for selecting against cells that have been transformed with a [http://ginkgobioworks.com/support/BioBrick_Assembly_Manual.pdf destination plasmid]. It worked perfectly, and if i might say, it looks smashingly in LB medium ;). |
|
•••••
|
We obtained this part from the Spring 2011 distribution plate. It expresses wonderfully in NEB10B cells from New England Biolabs. It does not express well in the fast-growing strain DH5-alpha Turbo, which we've found doesn't express transgenes very well in general. |
Team Westminster_UOW 2016
During our iGEM project we aimed to characterise two parts, BBa_J04450 which is a pSB1C3 plasmid present within the iGEM 2016 kit. We did a series of experiments testing the different expression rates it grows at in XL1-Blue and TOP10. We found that in XL1-blue it showed some but very little expression rate without IPTG in experiment 2.4. This may indicate that there is “leaky” expression of the RFP taking place. It was also found to grow better with the addition of 0.1mM IPTG (experiment 2.5) the RFP expression was much higher in XL1-Blue which was expected as XL1-Blue contains a LacI inhibitor encoded in its genomic DNA. However this raises the question as to why it showed a low level protein expression when un-induced by IPGT. It was found to grow extremely well in a TOP10 strain with or without IPTG which is expected as TOP10 does not contain the inhibitor molecule that is present within XL1-Blue. We grew the plasmid pSB3T5 (BBa_I52001) containing an RFP insert with a p15A replication origin and a tetracycline resistance gene, It showed protein expression at a low rate when un-induced and grown in TOP10 strain providing evidence for it being a low-medium copy number plasmid. We also determined that pSB1C3 (BBa_J04450) cannot grow in a co-culture of both XL1-blue and TOP10, the co-culture results showed little/no expression of the RFP therefore is ineffective at expressing in a co-culture.



Team iTesla-SoundBio 2017
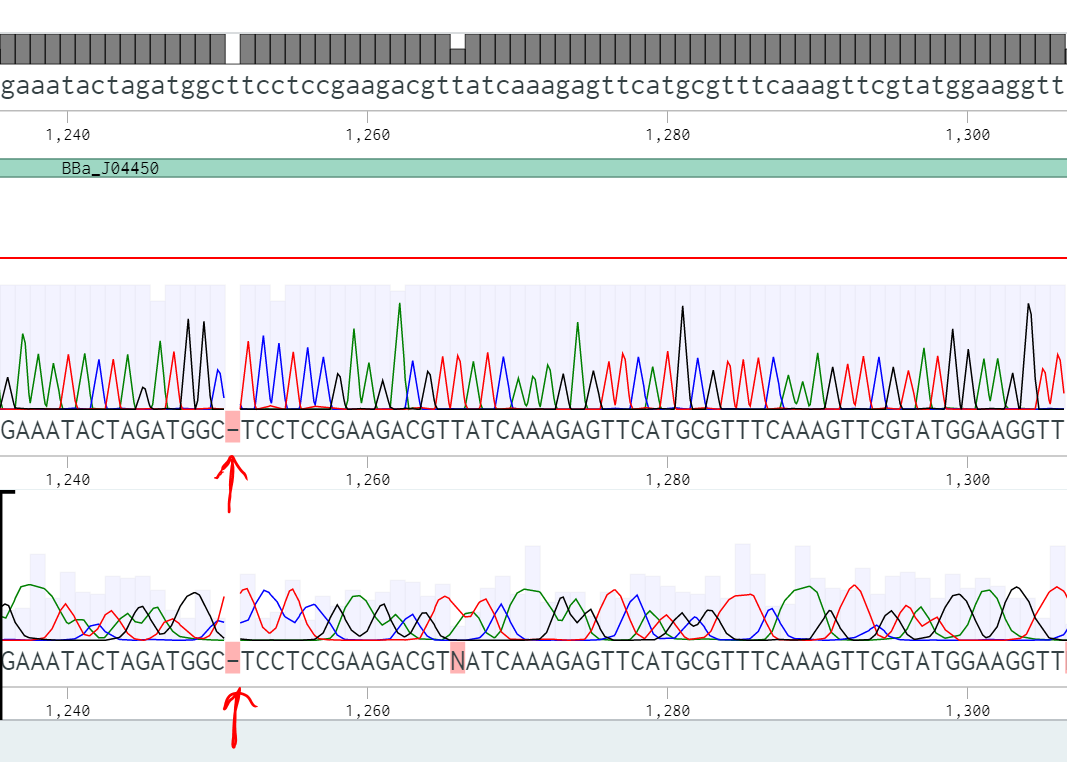
We used BBa_J04450, a popular biobrick among other iGEM teams as a vector to house our inserts. Encountering an abnormally high false positive rate with our transformations, we found this aspect of the plasmid worth investigating. Each step in the process (digesting, ligating, and transforming) compounds inefficiency, reducing the probability of a successful transformation. The false positive rate does not pose a problem for highly efficient events; however, for rare events where ligation efficiency is diminished, the chances of selecting a false positive increase dramatically. To characterize the false positive rate, we sequenced 8 colonies and determined the main causes of false positives.
In this context, we define a false positive result as a white colony that did not contain the desired insert based on sequencing data. Expanding on that, a negative result appeared as a red colony on the plate, and a ‘true positive’ was a white colony that, after sequencing, we learned contained the expected insert. Of the eight colonies we sequenced from two experiments, three were in fact true positives and five were false positives. Three of the false positives had a problem with their backbones and still had RFP (either undigested or religated BBa_J04450). Interestingly, two of the sequences only contained a fragment of the RFP gene.
The 3’ terminal deletion appears similar to a PstI cut site (one of our biobricking restriction enzymes), leading us to consider two hypotheses. First, the backbone plasmid had a mutation adding a PstI cut site in the middle of the RFP coding region. Second, the conditions of our digestion reaction may have elicited star activity allowing PstI to cut incorrectly.
There are solutions for these specific cases of false positives. One option is to run a more exhaustive purification gel. A longer run time and lower voltage would form tighter bands and would help differentiate between completely and partially digested backbones, decreasing inefficiency at this step in the cloning process. Another solution is to apply a silent mutation at base pair 1654, decreasing the odds of this sequence being recognized as a cut site.
Another false positive attributed to the backbone was caused by a frameshift mutation (Figure 2). These are unavoidable as the error occurred during PCR, so the only option would be to sequence minipreps after transformation, a precaution we already take.
We determined that the final source for false positives was insert related. There was a large-scale truncated inversion of the gene present in our backbone, and its placement infers such a large error in the process that we didn’t see value in investigating further.
Over all two rounds of our transformations and eight sequenced white colonies,three were true positives and five were false positives. Of those false positives, we determined that two plasmids suffered from pstI enzyme promiscuity (on the backbone), one plasmid incurred a frameshift mutation (also on the backbone), and one plasmid experienced an unprecedented truncated inversion with its insert. We determined a few ways to optimize efficiency, and decrease chances of backbone errors specifically; to run purification gels longer and at a lower voltage, therefore decreasing inefficiency during the digestion step, and to apply a silent mutation at base pair 1654, therefore eliminating the chances of a natural cut site being recognized there.
Team Westminster_UOW 2019
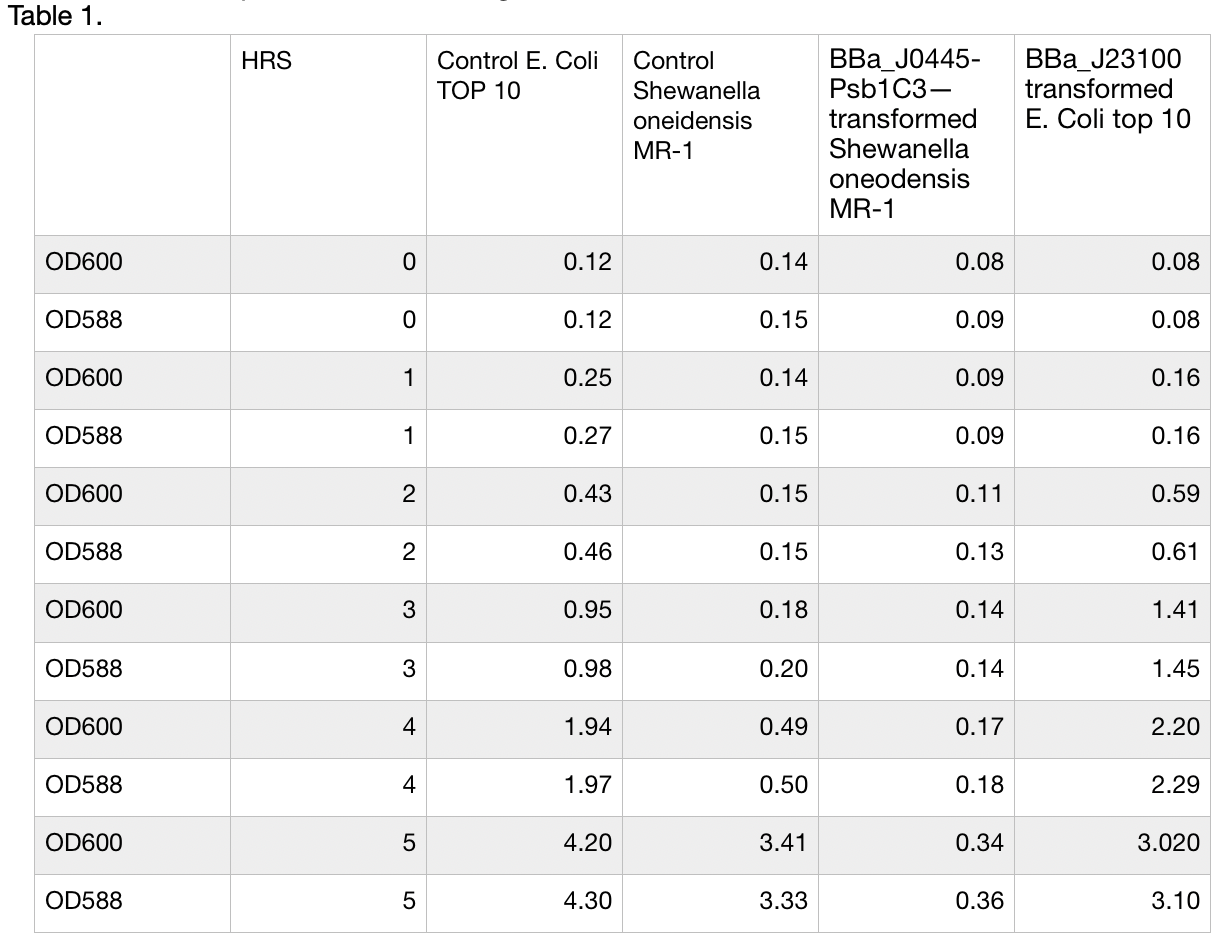
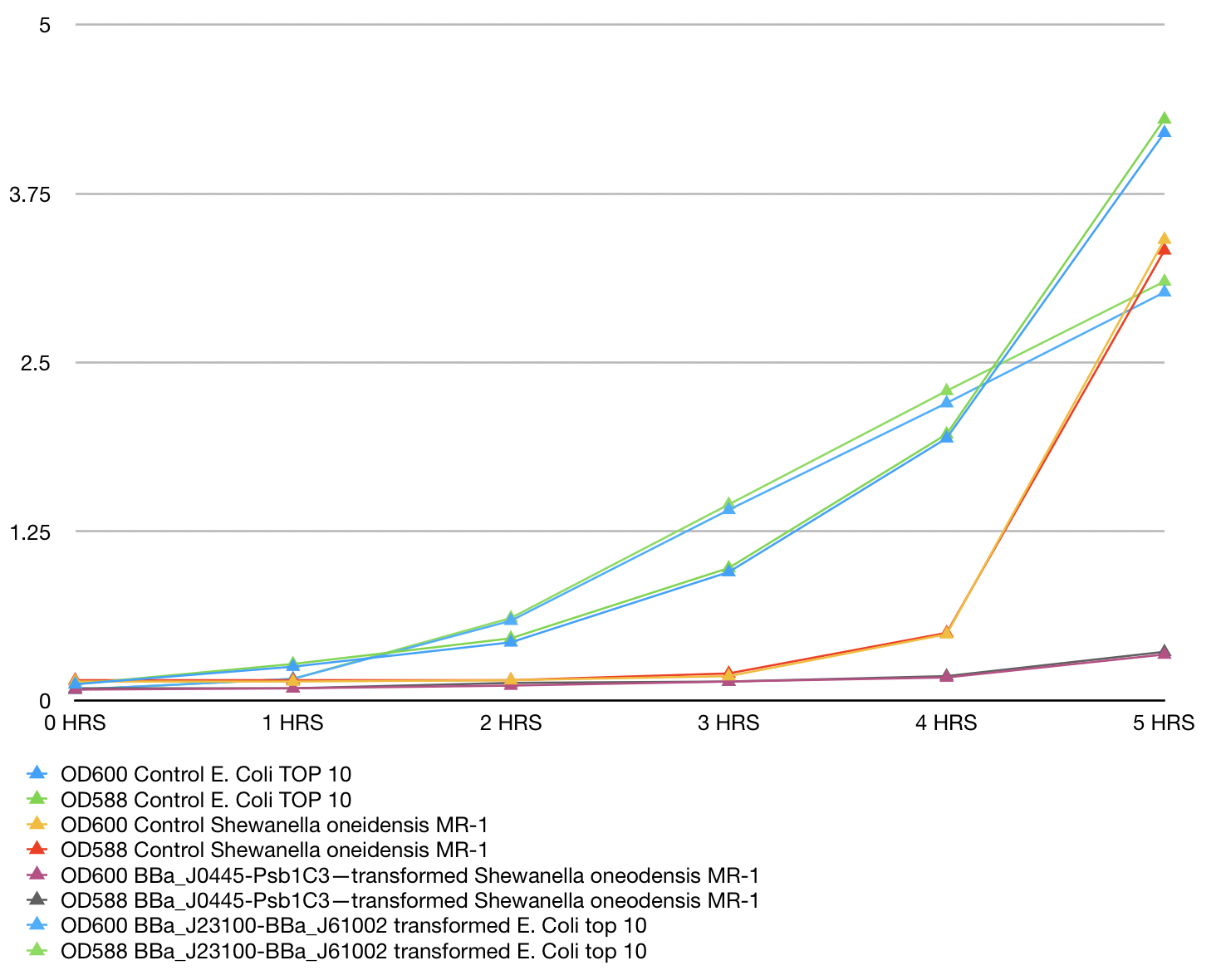
Growth and Red colour dynamics of BBa_J0445-Psb1C3—transformed Shewanella oneodensis MR-1. Shewanella oneodensis MR-1 was transformed via conjugation with donor E. coli TOP10 strain. Lb broth was inoculated with single colonies grown overnight and supplemented with 100ng/ul Chloramphenicol. mRFP and culture OD was measured at 588nm and 600 nm respectivaly. Measurements were taken for 5 consecutive hours of growth conditions: 30C, 200rpm. BBa_J23100 transformed E. Coli top 10 was used as reference, Untransformed E. Coli Top10 and Shewanella oneidensis MR-1 was used as control and reference. Measurements were take in triplicates using Nanodrop 200 Thermo scientific every hour and means were used to construct the plot. Data can be seen in table 1, plot can be seen in figure 1.
.
.
Team NAU-CHINA 2023 contribution: Effect of mRFP expression on the cell abundance measurements
Purpose & Design
In our contribution section, we decided to delve deeper into the investigation of the commonly used reporter gene, Red Fluorescent Protein (RFP). Red Fluorescent Proteins (RFPs) possess a strong absorption of light at the 600 nm wavelength, leading to a misrepresentation of cell optical density and consequently an underestimation of single-cell fluorescence. Thus, we selected BBa_J04450 from the iGEM part repository and used pSB1C3 as the vector to introduce it into Escherichia coli DH5α for expression, aiming to explore the influence of different light wavelengths on the deviation in measuring Red Fluorescent Protein (RFP) abundance in cells.

Fig. 1 The Plasmid Map of pSB1C3-J04450.
Experiment
1. We activated BBa_J04450 obtained from the kit and transformed it into E. coli DH5α, followed by overnight incubation at 37℃. The subsequent day, we chose 4 colonies and cultured them in 5 mL LB medium for approximately 12 hours. This genetic segment was expressed within the plasmid pSB1C3; consequently, both our LB agar plates and culture media were supplemented with chloramphenicol (50 μg/mL).
2. Used XbaI and SpeI enzymes to construct the empty vector pSB1C3.

Fig. 1 The Plasmid Map of pSB1C3.
3. The pSB1C3 plasmid was transformed into E. coli DH5α, followed by overnight incubation at 37℃. The next day, four colonies were selected and cultured in 5 mL LB medium for approximately 12 hours.
4. We prepared six fresh 50 mL LB media. Subsequently, we introduced overnight cultures of E. coli carrying pSB1C3-J04450 and pSB1C3 , respectively, into these media. The initial three media were inoculated with pSB1C3 E. coli, while the latter were pSB1C3-J04450 E. coli. These cultures were cultivated for sixteen hours. During the sampling process, we transferred 200 μL cultured samples into Costar 96 Flat Bottom Polystyrene Cat with black and transparent bottoms. Notably, each sample was subjected to three replicates within both the black and transparent groups.
5. After sampling, we promptly measured the data using an microplate reader (Tecan Spark) and conducted subsequent analysis.
Results
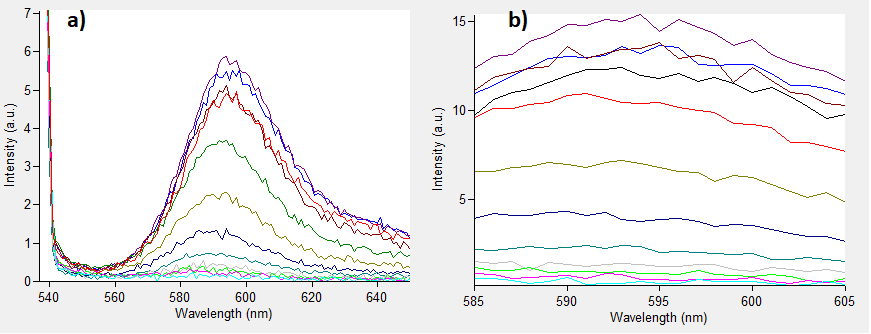
Among commonly used fluorescent proteins, the excitation wavelength for mRFP is situated around 585 nm (Fig. 2). Consequently, it exhibits a prominent absorption peak at 600 nm (Fig. 2), a standard wavelength used for measuring bacterial culture optical density (OD). This phenomenon leads to a significant overestimation of data when quantifying the abundance of bacteria expressing red fluorescent protein.

Fig. 2 Absorption spectra of mRFP1.
Initially, we measured the fluorescence levels of E. coli. Notably, the E. coli carrying pSB1C3-J04450 exhibited apronounced shift towards red fluorescence in the later stages. In the early stages, E. coli carrying pSB1C3-J04450 displayed fluorescence levels similar to those without it. However, after 12 hours, a substantial increase was observed, significantly surpassing the fluorescence levels of the E. coli carrying pSB1C3 (Fig. 3). This indicates the density of our experimental strain, which exhibits a distinct disparity compared to E. coli carrying pSB1C3.

Fig. 3 The Fluorescence of each culture.
We measured the OD values of the bacteria, revealing distinct differences between Escherichia coli strains carrying pSB1C3-J04450 and those carrying pSB1C3 at various wavelengths. Specifically, data obtained under 585 nm wavelength exhibited the most significant disparities between the two E. coli strains, while data acquired under 700 nm wavelength displayed less obvious distinctions (Fig. 4a-d). The increased disparity at 585 nm can be attributed to the absorption peak of mRFP occurring near this wavelength, thus making the data differences between the two strains more pronounced.Thus, the influence of RFP can be disregarded at the two longer wavelengths.
Furthermore, to gain further insight into the impact of RFP on E. coli OD values, we conducted deviation analyses relative to the baseline at 700 nm and performed linear function fitting for the data obtained at the other three wavelengths. It is evident that the data acquired at 660 nm exhibited minimal deviation, while the other two shorter wavelengths displayed a noticeable upward trend (Fig. 4E). At the 12-hour mark, the OD values obtained at 600 nm were nearly 15% higher than those unaffected by RFP. This illustrates the impact of mRFP expression on the measurement of bacterial density under wavelength at 600 nm. This serves as a valuable reference for future teams.

Fig. 4 The ODabs and Deviation Analyses a-d ODabs measurements were taken at wavelengths of 585 nm, 600 nm, 660 nm, and 700 nm. Due to the absorption peak of RFP at 585 nm, E. coli carrying pSB1C3-J04450 exhibited significantly higher data values at both 585 nm and 600 nm wavelengths compared to those carrying pSB1C3. However, data at 660 nm and 700 nm showed relatively minor differences. e: Using 700 nm as reference, bias analysis and function fitting were conducted for the data obtained at the other three wavelengths. In comparison to OD at 660 nm, the OD at 585 nm and 600 nm displayed a notable positive correlation, highlighting the significant impact of RFP expression on bacterial concentration measurements at 585 nm and 600 nm.
Reference
- Hecht A, Endy D, Salit M, et al. When wavelengths collide: bias in cell abundance measurements due to expressed fluorescent proteins[J]. ACS synthetic biology, 2016, 5(9): 1024-1027.
- Campbell R E, Tour O, Palmer A E, et al. A monomeric red fluorescent protein[J]. Proceedings of the National Academy of Sciences, 2002, 99(12): 7877-7882.
UNIQ38be9daa3368cba9-partinfo-00000010-QINU