Difference between revisions of "Part:BBa K4119001"
| (3 intermediate revisions by the same user not shown) | |||
| Line 8: | Line 8: | ||
In order to test the function of these chromoproteins, we constructed Pet-29a(+)-tsPurple. | In order to test the function of these chromoproteins, we constructed Pet-29a(+)-tsPurple. | ||
<Br> | <Br> | ||
| − | + | The expression was induced by T7 RNA polymerase provided by host cells. E.coil BL21 was used as receptor bacteria to express the protein, and then the fusion protein with histidine was purified by Nickle-affinity chromatography. If this chromoprotein are functional, we can observe the correct band through plasmid PCR, agarose gel electrophoresis, and the obvious color of the colony. After a series of purification operations, the correct band was observed by SDS-PAGE. Finally, use Mass Spectrometer to detect the exact molecular weight of the protein for final confirmation. | |
Our experiment results matched the general expected trend and data. | Our experiment results matched the general expected trend and data. | ||
| Line 15: | Line 15: | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| − | |||
| − | |||
| − | |||
| Line 24: | Line 21: | ||
<partinfo>BBa_K4119001 parameters</partinfo> | <partinfo>BBa_K4119001 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | ===Results=== | ||
| + | <Br> | ||
| + | Hoping to make a useful contribution for future iGEM teams,we completed the experimental characterization of existing parts tsPurple, purple chromoprotein(incl RBS) and meffBlue, blue chromoprotein and provided new documents for both of them. | ||
| + | The tsPurple sequence (Part:BBa_K1033905) optimized for E. coli was incorporated into plasmid pET-29a(+), transformed into E. coli BL21 for characterization and measurement. We provided tsPurple with results and data based on protein expression and purification, TOF-Mass spectrometry, and Swiss-model. | ||
| + | <Br> | ||
| + | <Br> | ||
| + | Methods:<Br> | ||
| + | SDS-PAGE, ultramicro spectrophotometer ,TOF-Mass Spectrometry, and Swiss-Model. | ||
| + | <Br> | ||
| + | <Br> | ||
| + | Results: | ||
| + | [[Image:tsPurple.png|500px|center]] | ||
| + | Fig.1 The fermentation broth of tsPurple Fig.2 Proteins after secondary ultrafiltration<Br> | ||
| + | <Br> | ||
| + | Conclusion: The cell pellet was collected by harvesting 50mL culture after 24h of induction followed by centrifugation at 4 degrees and 6000 rpm for 10min. Then, we performed ultrasonic disruption and collected the supernatant after centrifugation. The protein was purified and collected through ultrafiltration and affinity chromatography. | ||
| + | <Br> | ||
| + | [[Image:ts_SDS_PAGE.png|500px|center]] | ||
| + | Fig.3 SDS-PAGE of the chromoprotein tsPurple<Br> | ||
| + | 1· tsPurple- The culture without IPTG induction. <Br> | ||
| + | 2· tsPurple- Supernatant without IPTG induction after sonication.<Br> | ||
| + | 3· tsPurple- The sedimentation without IPTG induction and after sonication.<Br> | ||
| + | 4· tsPurple- Supernatant sample without IPTG induction after sonication.<Br> | ||
| + | 5· tsPurple- The sedimentation after IPTG induction and ultrasound.<Br> | ||
| + | 6· tsPurple- The culture after IPTG induction.<Br> | ||
| + | 7· tsPurple- Protein sample after the ultrafiltration (diluted 5 times).<Br> | ||
| + | 8. tsPurple- Protein after GST affinity chromatography.<Br> | ||
| + | 9· tsPurple- Purified protein sample.<Br> | ||
| + | <Br> | ||
| + | Conclusion: The protein gel preliminarily proved that the molecular mass of the tsPurple protein was correct, which is consistent with the expected molecular mass of tsPurple protein (the molecular mass of amajLime protein is about 26.9 kDa). Compared with lane 5, 6 and 7, lane 1, 2,3 and 4 indicate that more tsPurple protein can be obtained with IPTG induction. As is shown in lane 8, the concentration of protein was increased after ultrafiltration concentration. Lane 9 shows that the purification effect of protein after nickel affinity chromatography was better, and the impurity protein was less than before affinity chromatography. In conclusion, it can be seen that our expression and purification strategy is effective. | ||
| + | <Br> | ||
| + | [[Image:ts_T.png|500px|center]] | ||
| + | Fig. 4 concentration of the tsPurple protein samples<Br> | ||
| + | Conclusion:We used the ultramicro spectrophotometer to measure the concentration of tsPurple protein. The concentration of tsPurple samples was 0.7533 mg/ml | ||
| + | <Br> | ||
| + | [[Image:ts_TOF_MS.png|500px|center]] | ||
| + | Fig.5 TOF MS of tsPurple.<Br> | ||
| + | Conclusion: We performed a Time of Flight Mass Spectrometer on the purified HIS-tagged tsPurple protein. The predicted molecular mass of this protein is about 26.9KDa. The result of TOF-Mass Spectrometry showed that the specific molecular mass of tsPurple protein is 26.27Da (the value of the sharpest peak is shown as the molecular mass of tsPurple protein).<Br> | ||
| + | <Br> | ||
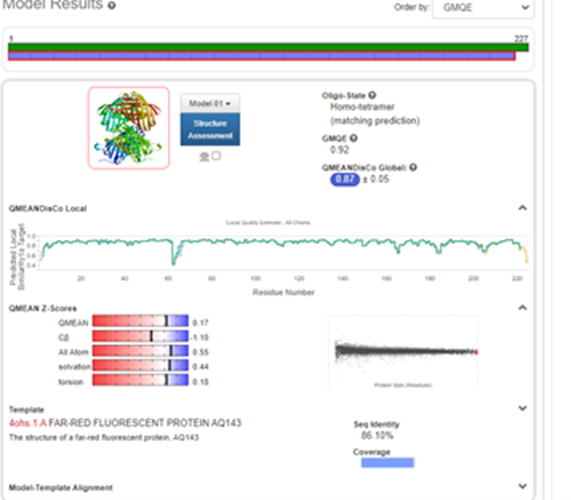
| + | Structural modeling results of the tsPurple protein based on Swiss-Model<Br> | ||
| + | [[Image:ts_model_p1.png|500px|center]] | ||
| + | [[Image:ts_model_p2.png|500px|center]] | ||
| + | [[Image:ts_model_p3.png|500px|center]] | ||
| + | Fig.6-1 The results of the homology and structural modelling protein tsPurple.<Br> | ||
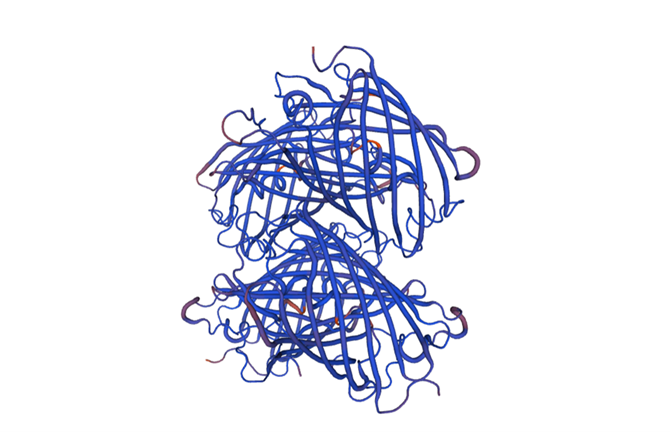
| + | [[Image:ts_model_3d.png|500px|center]] | ||
| + | Fig.6-2 The 3D model of the homology and structural modelling protein tsPurple.<Br> | ||
| + | Conclusion: We used Swiss-Model to simulate the three-dimensional structure of tsPurple protein. The above figures showed the modeling result of Swiss-Model.<Br> | ||
| + | |||
| + | <!-- --> | ||
| + | <span class='h3bb'>Sequence and Features</span> | ||
| + | <partinfo>BBa_K4119001 SequenceAndFeatures</partinfo> | ||
Latest revision as of 15:39, 12 October 2022
T7 promoter-lacO-tsPurple-6xHisTag-T7 terminator
Hoping to make a useful contribution for future iGEM teams,we completed the experimental characterization of existing part, tsPurple, purple chromoprotein(incl RBS)(BBa_K1033905) and provided new documents for this part.
Our team was very interested in the characterization of this chromoprotein and carried out the design and implementation of related experiments.
In order to test the function of these chromoproteins, we constructed Pet-29a(+)-tsPurple.
The expression was induced by T7 RNA polymerase provided by host cells. E.coil BL21 was used as receptor bacteria to express the protein, and then the fusion protein with histidine was purified by Nickle-affinity chromatography. If this chromoprotein are functional, we can observe the correct band through plasmid PCR, agarose gel electrophoresis, and the obvious color of the colony. After a series of purification operations, the correct band was observed by SDS-PAGE. Finally, use Mass Spectrometer to detect the exact molecular weight of the protein for final confirmation.
Our experiment results matched the general expected trend and data.
Results
Hoping to make a useful contribution for future iGEM teams,we completed the experimental characterization of existing parts tsPurple, purple chromoprotein(incl RBS) and meffBlue, blue chromoprotein and provided new documents for both of them.
The tsPurple sequence (Part:BBa_K1033905) optimized for E. coli was incorporated into plasmid pET-29a(+), transformed into E. coli BL21 for characterization and measurement. We provided tsPurple with results and data based on protein expression and purification, TOF-Mass spectrometry, and Swiss-model.
Methods:
SDS-PAGE, ultramicro spectrophotometer ,TOF-Mass Spectrometry, and Swiss-Model.
Results:
Fig.1 The fermentation broth of tsPurple Fig.2 Proteins after secondary ultrafiltration
Conclusion: The cell pellet was collected by harvesting 50mL culture after 24h of induction followed by centrifugation at 4 degrees and 6000 rpm for 10min. Then, we performed ultrasonic disruption and collected the supernatant after centrifugation. The protein was purified and collected through ultrafiltration and affinity chromatography.
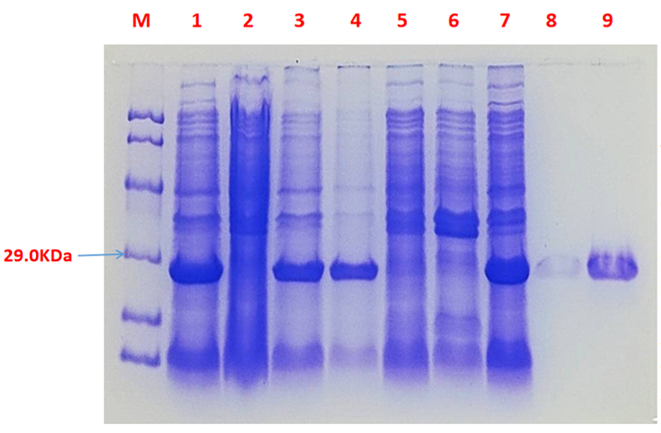
Fig.3 SDS-PAGE of the chromoprotein tsPurple
1· tsPurple- The culture without IPTG induction.
2· tsPurple- Supernatant without IPTG induction after sonication.
3· tsPurple- The sedimentation without IPTG induction and after sonication.
4· tsPurple- Supernatant sample without IPTG induction after sonication.
5· tsPurple- The sedimentation after IPTG induction and ultrasound.
6· tsPurple- The culture after IPTG induction.
7· tsPurple- Protein sample after the ultrafiltration (diluted 5 times).
8. tsPurple- Protein after GST affinity chromatography.
9· tsPurple- Purified protein sample.
Conclusion: The protein gel preliminarily proved that the molecular mass of the tsPurple protein was correct, which is consistent with the expected molecular mass of tsPurple protein (the molecular mass of amajLime protein is about 26.9 kDa). Compared with lane 5, 6 and 7, lane 1, 2,3 and 4 indicate that more tsPurple protein can be obtained with IPTG induction. As is shown in lane 8, the concentration of protein was increased after ultrafiltration concentration. Lane 9 shows that the purification effect of protein after nickel affinity chromatography was better, and the impurity protein was less than before affinity chromatography. In conclusion, it can be seen that our expression and purification strategy is effective.
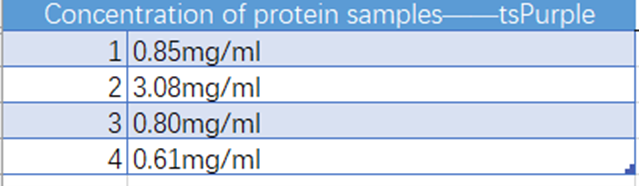
Fig. 4 concentration of the tsPurple protein samples
Conclusion:We used the ultramicro spectrophotometer to measure the concentration of tsPurple protein. The concentration of tsPurple samples was 0.7533 mg/ml
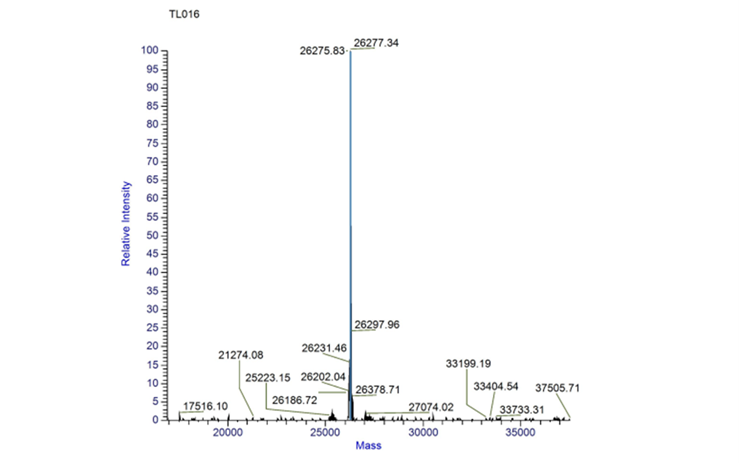
Fig.5 TOF MS of tsPurple.
Conclusion: We performed a Time of Flight Mass Spectrometer on the purified HIS-tagged tsPurple protein. The predicted molecular mass of this protein is about 26.9KDa. The result of TOF-Mass Spectrometry showed that the specific molecular mass of tsPurple protein is 26.27Da (the value of the sharpest peak is shown as the molecular mass of tsPurple protein).
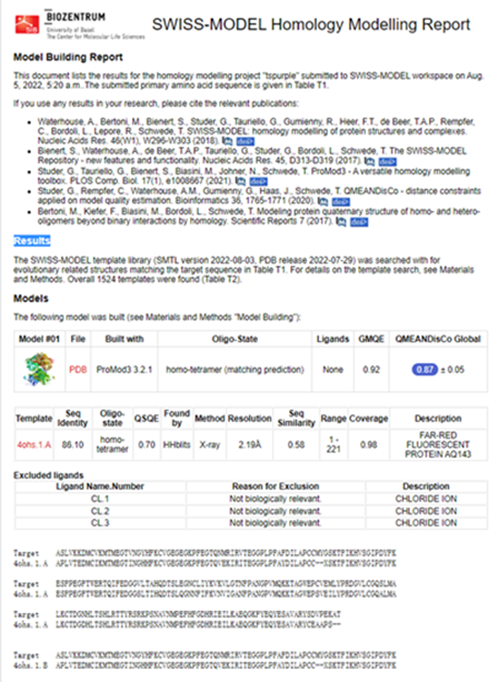
Structural modeling results of the tsPurple protein based on Swiss-Model
Fig.6-1 The results of the homology and structural modelling protein tsPurple.
Fig.6-2 The 3D model of the homology and structural modelling protein tsPurple.
Conclusion: We used Swiss-Model to simulate the three-dimensional structure of tsPurple protein. The above figures showed the modeling result of Swiss-Model.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]