Difference between revisions of "Part:BBa K4214003"
(→SDS-PAGE) |
|||
| Line 31: | Line 31: | ||
===SDS-PAGE=== | ===SDS-PAGE=== | ||
A production culture was set up using BL21 ''E. coli'' and induced with 0.5 mM IPTG and 1 mM IPTG once their ODs reached 0.5. The cultures were left overnight at 30°C for protein production. The cells were then harvested, pelleted and purified using a HisPur Cobalt resin. The SDS-PAGE analysis of the production process is shown on the right. | A production culture was set up using BL21 ''E. coli'' and induced with 0.5 mM IPTG and 1 mM IPTG once their ODs reached 0.5. The cultures were left overnight at 30°C for protein production. The cells were then harvested, pelleted and purified using a HisPur Cobalt resin. The SDS-PAGE analysis of the production process is shown on the right. | ||
| − | |||
[[Image:BBa K4214003 SDSPAGE.png|600px|thumb|center|Fig.: ''SDS-PAGE of BBa K4214003 part for protein production. CP: Cell pellet, FL: Filtered lysate'']] | [[Image:BBa K4214003 SDSPAGE.png|600px|thumb|center|Fig.: ''SDS-PAGE of BBa K4214003 part for protein production. CP: Cell pellet, FL: Filtered lysate'']] | ||
Latest revision as of 22:46, 7 October 2023
hGIF with LacI promotor and 6xHis-tag
LacI (BBa_J04500) and Human GIF with His6-tag. This part is a composite part from BBa_K4214002 with the addition of LacI sequence (BBa_J04500). The LacI sequence is used in order to induce our protein expression with IPTG, and the 6xHis-tag was used for protein purification.
Usage and Biology
- NCBI page of the human gastric intrinsic factor [1]
- This part of hGIF does not contain the native signal peptide (AA1-18)
- The LacI promotor is described in part BBa_J04500
Characterization
Agarose Gel
Restriction Digestion of the pSB1C3 with LacI (BBa_J04500) using the SpeI and PstI restriction enzymes and restriction digestion of the Human GIF with His6-tag (BBa_K4214002)by using the XbaI and PstI. After the restriction digestion we proceed with the ligation of the two parts creating the BBa K4214003 part.
SDS-PAGE
A production culture was set up using BL21 E. coli and induced with 0.5 mM IPTG and 1 mM IPTG once their ODs reached 0.5. The cultures were left overnight at 30°C for protein production. The cells were then harvested, pelleted and purified using a HisPur Cobalt resin. The SDS-PAGE analysis of the production process is shown on the right.
Western Blot
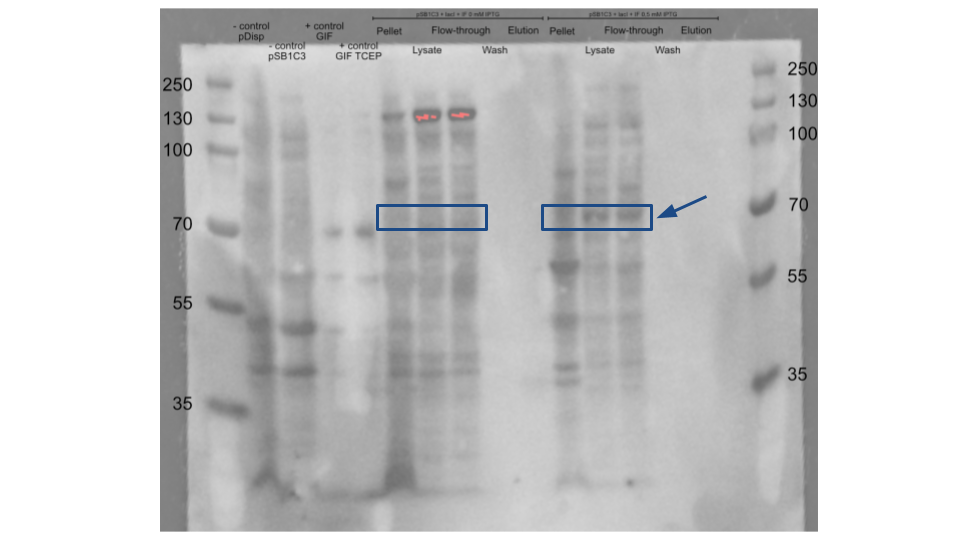
In order to implement our idea and have our protein into a pill, we also attempted to produce and purify the wtIF. The same procedure will be used to produce and purify the best mutant . For that reason, we used the pSB1C3 Biobrick plasmid with the LacI promotor (BBa_J04500). This promotor is inducible by IPTG, which will drive the protein expression, and the gene of interest, in our case wtIF. Protein production was initiated using this plasmid.As a negative control we used the pDisplay™ mammalian expression vector and the pSB1C3+LacI without the wtIF., A recombinant Gastric Intrinsic Factor protein (From Sinobiological:Cat: 13544-H08H) was used as a positive control for migration size and antibody binding. . After the induction of the protein expression with IPTG, we purified our protein using HisPure Cobalt Resin. During the purification we collected the cell pellet, the flow through and the eluted part of the purification column. We loaded all of our samples in the SDS-page and followed it with a Western Blot. We used the epitope unspecific Intrinsic Factor Rabbit primary antibody [2] (1:100) and Anti-Rabbit HRP(1:5000) as a secondary antibody. The wtIF expression was seen at the inducer concentration of 0.5 mM. The wtIF is detected around 55-70 kDa in the Lysate and Flow-through of the 0.5 mM IPTG samples but not in the Eluted parts of the purification.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 485
Illegal BsaI.rc site found at 1165
Illegal SapI site found at 1155