Difference between revisions of "Part:BBa K4365006"
Svetlana ia (Talk | contribs) |
Svetlana ia (Talk | contribs) (→Usage and Biology) |
||
| (19 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K4365006 short</partinfo> | <partinfo>BBa_K4365006 short</partinfo> | ||
| − | MPGI is a protein found in ''Magnaporthe grisea'' and belongs to a family of highly secreted proteins called hydrophobins. The signal peptide from the protein | + | MPGI is a protein found in ''Magnaporthe grisea'' and belongs to a family of highly secreted proteins called hydrophobins. The signal peptide from the protein led to <html><a href="https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365020">turboRFP</a></html> secretion in ''Saccharomyces cerevisiae''. |
{| class="wikitable" style="margin-left: auto; margin-right: auto; border: none; width:600px;" | {| class="wikitable" style="margin-left: auto; margin-right: auto; border: none; width:600px;" | ||
| Line 60: | Line 60: | ||
| − | |||
===Usage and Biology=== | ===Usage and Biology=== | ||
| + | Secretion signal peptides can be fused to a protein to direct it through the secretory pathway out of the cell <ref name="2albert">Alberts, B. (2015) Molecular Biology of the Cell. 6th Edition, Garland Science, Taylor and Francis Group, New York.</ref>. Such secretion of recombinant proteins facilitates the protein purification process and the removal of GMOs <ref name="1kirsten">Kirsten Kottmeier, Kai Ostermann, Thomas Bley, Gerhard Rödel (2011) Hydrophobin signal sequence mediates efficient secretion of recombinant proteins in Pichia pastoris, Appl Microbiol Biotechnol 91, pages133–141, https://doi.org/10.1007/s00253-011-3246-y</ref>. However, commonly used signal sequences like the ''S. cerevisiae'' alpha-factor prepro-peptide <html><a href="https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365019">K4365019</a></html> and the HSP150, are very long <ref name="1kirsten" /><ref>Russo P, Kalkkinen N, Sareneva H, Paakkola J, Makarow M. A heat shock gene from Saccharomyces cerevisiae encoding a secretory glycoprotein. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3671-5. doi: 10.1073/pnas.89.9.3671. Erratum in: Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8857. PMID: 1570286; PMCID: PMC525552.</ref> which makes their use in genetic constructs limited. <!--This is a part to change for other peptides--> '''K4365006''' as a part of '''the collection of signal peptides''' represents a shorter sequence that could be inserted into the gene of interest simply by PCR and could display higher secretion efficiency. <!--This is a part to change citations for other peptides--> | ||
| − | < | + | The sequences of the hydrophobic signal peptide was collected from literature <ref>N J Talbot, D J Ebbole, J E Hamer (1993) Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea, The Plant Cell, Volume 5, Issue 11, Pages 1575–1590, https://doi.org/10.1105/tpc.5.11.1575</ref> and was extracted via analysis of their sequence using the SignalP - 5.0 signal peptide predictor tool <ref>José Juan Almagro Armenteros et al. (2019) SignalP 5.0 improves signal peptide predictions using deep neural networks Nature Biotechnology, 37, 420-423, doi: 10.1038/s41587-019-0036-z </ref>. |
| + | <!--This is a part to change citations for other peptides--> | ||
| + | Identified sequence was codon-optimized for ''S. cerevisiae'' using the codon optimization tool on GenScript. Then it was synthesized by IDT as a gBlock together with <html>a flexible glycine linker (<a href="https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365005">K4365005</a>) and the turboRFP gene (<a href="https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365020">K4365020</a>)</html> <ref>Merzlyak, E., Goedhart, J., Shcherbo, D. et al. (2007) Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat Methods 4, 555–557 (2007). https://doi.org/10.1038/nmeth1062</ref>. | ||
| + | <!--This is a part to change citations for other peptides--> | ||
| + | ====Hydrophobins==== | ||
| + | Hydrophobins are a group of small (~100 amino acids) proteins that are expressed in ascomycetes and basidiomycetes, which are very efficiently secreted <ref name="1kirsten" />. | ||
| + | |||
| + | After secretion from the fungus, they can assemble on the cell walls to form a hydrophobic coating. For this reason, the functions of hydrophobins are related to cell surface activity. For example, they can cover hyphae so the surface tension can be lowered and the hyphae can breach the water/air interface, thus escaping from the aqueous environment and growing into the aerial hyphae <ref name="5khal">Khalesi, M., Gebruers, K. & Derdelinckx, G. (2015) Recent Advances in Fungal Hydrophobin Towards Using in Industry, Protein J, 34, 243–255 . https://doi.org/10.1007/s10930-015-9621-2 </ref>. They also mediate the adhesion of the hyphae to hydrophobic surfaces such as in the case of pathogen-host interaction or symbiosis, as observed in lichens and in ectomycorrhizae (the symbiotic relationship between a fungal symbiont and the roots of a plant) <ref name="5khal" />. Hydrophobins also form a hydrophobic sheath to protect the surface of conidia, spores, and caps of fungi against wetting and desiccation <ref name="5khal" />. | ||
| + | |||
| + | ====Signal peptide secretion screening==== | ||
| + | |||
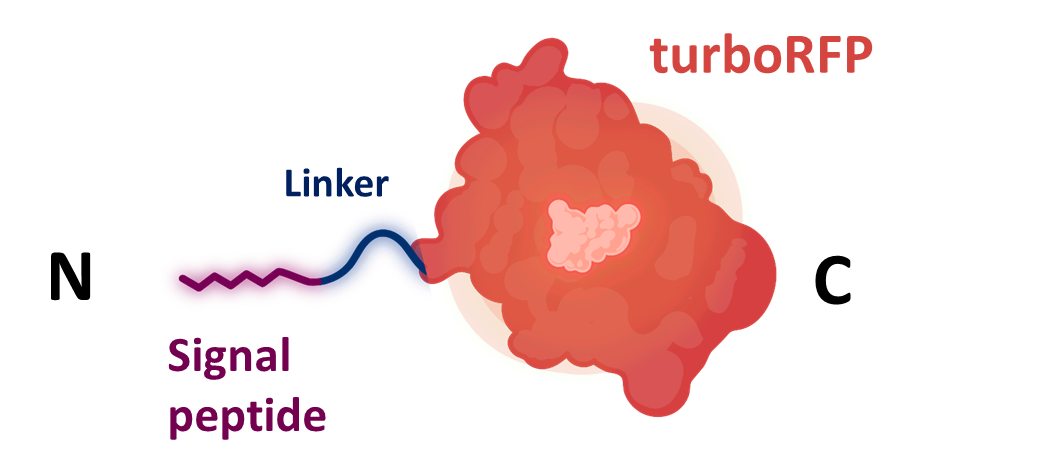
| + | Signal peptide siquence was cloned together with turboRFP <html><a href="https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365020">K4365020</a> into the level 1 shuttle plasmid <a href="https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365022">K4365022</a></html> (Figure 1). | ||
| + | |||
| + | [[File:BBa_K4365006_screening.png|500px|center|thumb|Figure 1: fusion protein used for the secretion assays composed of a signal peptide, a flexible linker, and the turboRFP fluorescent protein. Created with Biorender.com.]] | ||
| + | |||
| + | After the transformation with the shuttle plasmid, the yeast colonies were grown on W0 medium plates supplemented with histidine, leucine, and methionine and were used for secretion assays in flasks and in a 48-well plate. The α-mating factor pre-pro signal peptide <html><a href="https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365019">K4365019</a></html> was used as a positive control as it has been shown to be able to secrete proteins <ref name="19penas">Peñas M. M. et al. (1998) Identification, characterization, and In situ detection of a fruit-body-specific hydrophobin of Pleurotus ostreatus, Appl Environ Microbiol. 64(10):4028-34. doi: 10.1128/AEM.64.10.4028-4034.1998. PMID: 9758836; PMCID: PMC106595.</ref>. A turboRFP without signal peptide was employed as a negative control to account for the amount of free turboRFP present in the media due to cell lysis. | ||
| + | |||
| + | '''Secretion measurement protocols:''' | ||
| + | *[https://parts.igem.org/Part:BBa_K4365021#Secretion_assay_in_a_flask_protocol flask protocol] | ||
| + | *[https://parts.igem.org/Part:BBa_K4365021#Secretion_assay_in_a_48-well_plate_protocol 48-well plate protocol] | ||
| + | |||
| + | <!--This part different between sps--> | ||
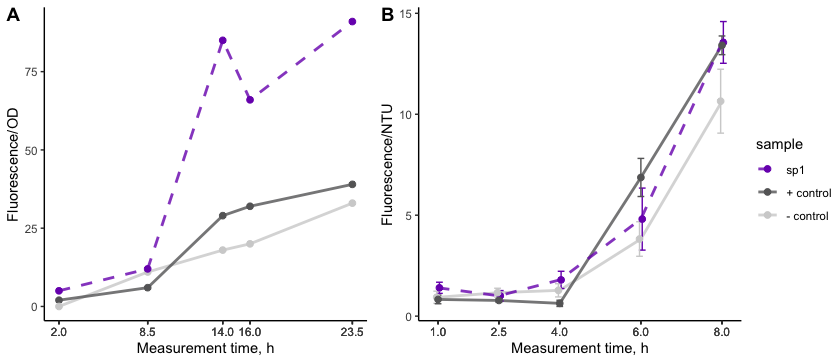
| + | The first secretion experiment was conducted in flasks and showed (Figure 2A) that cell lysis was low as indicated by the low fluorescence of the negative control. Moreover, secretion with K4365006 outperformed the positive control. | ||
| + | |||
| + | In order to have consistent results and ensure confidence we set up biological triplicates. For this, turboRFP secretion was measured using 48-well plate approach. Cells with induced expression were pipetted into the wells of the plate and grown overnight. Starting at 1 hour after induction, samples were taken and the growth rate was monitored by measuring turbidity (NTU) and the development of fluorescence in the supernatant was measured on a plate reader. With this data K4365006 still showed secretion but without a dramatical difference to the positive control (Figure 2B). | ||
| + | <!--This part different between sps--> | ||
| + | |||
| + | [[File:BBa_K4365006_secretion.png|600px|center|thumb| Figure 2: '''A''' secretion by signal peptides in flasks. | ||
| + | '''B''' secretion assay in 48-well plate with an initial cell density of 1 OD. The canonical α-mating factor pre-pro signal peptide <ref name="19penas" /> (dark grey + control) was used as a positive control as it has been shown to be able to secrete proteins. A turboRFP without signal peptide was employed as a negative control to account for cell lysis (light grey, - control). The signal corresponding to K4365006 is depicted as a dashed line. Error bars represent mean ± SD.]] | ||
| + | |||
| + | ===References=== | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
<partinfo>BBa_K4365006 parameters</partinfo> | <partinfo>BBa_K4365006 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
Latest revision as of 21:42, 11 October 2022
Signal peptide of MPGI from Magnaporthe grisea
MPGI is a protein found in Magnaporthe grisea and belongs to a family of highly secreted proteins called hydrophobins. The signal peptide from the protein led to turboRFP secretion in Saccharomyces cerevisiae.
| ID | Source | Species | Prediction coefficient [1] | turboRFP secretion | |
|---|---|---|---|---|---|
| sp1 | BBa_K4365006 | MPGI | Magnaporthe grisea | 0.9562 | Yes |
| sp2 | BBa_K4365007 | RodA | Aspergillus nidulans | 0.9553 | Yes |
| sp3 | BBa_K4365008 | HYPI | Aspergillus fumigatus | 0.9703 | No |
| sp4 | BBa_K4365009 | SsgA | Metarhizium anisopliae | 0.9126 | No |
| sp5 | BBa_K4365010 | HCF-4 | Cladosporium fulvum | 0.9126 | Yes |
| sp6 | BBa_K4365025 | HCF-5 | Cladosporium fulvum | 0.9234 | NA |
| sp7 | BBa_K4365026 | CCG-2 | Neurospora crassa | 0.9566 | NA |
| sp8 | BBa_K4365011 | HCF-1 | Cladosporium fulvum | 0.6850 | Yes |
| sp9 | BBa_K4365027 | HCF-2 | Cladosporium fulvum | 0.8144 | NA |
| sp10 | BBa_K4365012 | HCF-3 | Cladosporium fulvum | 0.7934 | No |
| sp11 | BBa_K4365013 | SC3 | Schizophyllum commune | 0.6327 | No |
| sp12 | BBa_K4365014 | ABH3 | Agaricus bisporus | 0.9458 | Yes |
| sp13 | BBa_K4365028 | COH1 | Coprinus cinereus | 0.8545 | NA |
| sp14 | BBa_K4365015 | FBH1 | Pleurotus ostreatus | 0.3367 | Yes |
| sp15 | BBa_K4365016 | Aa-PRI2 | Agrocybe aegerita | 0.6356 | Yes |
| sp16 | BBa_K4365033 | Hyd-Pt1 | Pisolithus tinctorius | 0.8352 | NA |
| sp17 | BBa_K4365017 | HFBI | Trichoderma reesei | 0.9851 | Yes |
| sp18 | BBa_K4365018 | HFBII | Trichoderma reesei | 0.6862 | Yes |
| sp19 | BBa_K4365019 | α-mating factor | Saccharomyces cerevisiae | 0.9988 | Yes |
- ↑ José Juan Almagro Armenteros et al. (2019) SignalP 5.0 improves signal peptide predictions using deep neural networks Nature Biotechnology, 37, 420-423, doi: 10.1038/s41587-019-0036-z
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
Secretion signal peptides can be fused to a protein to direct it through the secretory pathway out of the cell [1]. Such secretion of recombinant proteins facilitates the protein purification process and the removal of GMOs [2]. However, commonly used signal sequences like the S. cerevisiae alpha-factor prepro-peptide K4365019 and the HSP150, are very long [2][3] which makes their use in genetic constructs limited. K4365006 as a part of the collection of signal peptides represents a shorter sequence that could be inserted into the gene of interest simply by PCR and could display higher secretion efficiency.
The sequences of the hydrophobic signal peptide was collected from literature [4] and was extracted via analysis of their sequence using the SignalP - 5.0 signal peptide predictor tool [5].
Identified sequence was codon-optimized for S. cerevisiae using the codon optimization tool on GenScript. Then it was synthesized by IDT as a gBlock together with a flexible glycine linker (K4365005) and the turboRFP gene (K4365020) [6].
Hydrophobins
Hydrophobins are a group of small (~100 amino acids) proteins that are expressed in ascomycetes and basidiomycetes, which are very efficiently secreted [2].
After secretion from the fungus, they can assemble on the cell walls to form a hydrophobic coating. For this reason, the functions of hydrophobins are related to cell surface activity. For example, they can cover hyphae so the surface tension can be lowered and the hyphae can breach the water/air interface, thus escaping from the aqueous environment and growing into the aerial hyphae [7]. They also mediate the adhesion of the hyphae to hydrophobic surfaces such as in the case of pathogen-host interaction or symbiosis, as observed in lichens and in ectomycorrhizae (the symbiotic relationship between a fungal symbiont and the roots of a plant) [7]. Hydrophobins also form a hydrophobic sheath to protect the surface of conidia, spores, and caps of fungi against wetting and desiccation [7].
Signal peptide secretion screening
Signal peptide siquence was cloned together with turboRFP K4365020 into the level 1 shuttle plasmid K4365022 (Figure 1).
After the transformation with the shuttle plasmid, the yeast colonies were grown on W0 medium plates supplemented with histidine, leucine, and methionine and were used for secretion assays in flasks and in a 48-well plate. The α-mating factor pre-pro signal peptide K4365019 was used as a positive control as it has been shown to be able to secrete proteins [8]. A turboRFP without signal peptide was employed as a negative control to account for the amount of free turboRFP present in the media due to cell lysis.
Secretion measurement protocols:
The first secretion experiment was conducted in flasks and showed (Figure 2A) that cell lysis was low as indicated by the low fluorescence of the negative control. Moreover, secretion with K4365006 outperformed the positive control.
In order to have consistent results and ensure confidence we set up biological triplicates. For this, turboRFP secretion was measured using 48-well plate approach. Cells with induced expression were pipetted into the wells of the plate and grown overnight. Starting at 1 hour after induction, samples were taken and the growth rate was monitored by measuring turbidity (NTU) and the development of fluorescence in the supernatant was measured on a plate reader. With this data K4365006 still showed secretion but without a dramatical difference to the positive control (Figure 2B).
References
- ↑ Alberts, B. (2015) Molecular Biology of the Cell. 6th Edition, Garland Science, Taylor and Francis Group, New York.
- ↑ 2.0 2.1 2.2 Kirsten Kottmeier, Kai Ostermann, Thomas Bley, Gerhard Rödel (2011) Hydrophobin signal sequence mediates efficient secretion of recombinant proteins in Pichia pastoris, Appl Microbiol Biotechnol 91, pages133–141, https://doi.org/10.1007/s00253-011-3246-y
- ↑ Russo P, Kalkkinen N, Sareneva H, Paakkola J, Makarow M. A heat shock gene from Saccharomyces cerevisiae encoding a secretory glycoprotein. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3671-5. doi: 10.1073/pnas.89.9.3671. Erratum in: Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8857. PMID: 1570286; PMCID: PMC525552.
- ↑ N J Talbot, D J Ebbole, J E Hamer (1993) Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea, The Plant Cell, Volume 5, Issue 11, Pages 1575–1590, https://doi.org/10.1105/tpc.5.11.1575
- ↑ José Juan Almagro Armenteros et al. (2019) SignalP 5.0 improves signal peptide predictions using deep neural networks Nature Biotechnology, 37, 420-423, doi: 10.1038/s41587-019-0036-z
- ↑ Merzlyak, E., Goedhart, J., Shcherbo, D. et al. (2007) Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat Methods 4, 555–557 (2007). https://doi.org/10.1038/nmeth1062
- ↑ 7.0 7.1 7.2 Khalesi, M., Gebruers, K. & Derdelinckx, G. (2015) Recent Advances in Fungal Hydrophobin Towards Using in Industry, Protein J, 34, 243–255 . https://doi.org/10.1007/s10930-015-9621-2
- ↑ 8.0 8.1 Peñas M. M. et al. (1998) Identification, characterization, and In situ detection of a fruit-body-specific hydrophobin of Pleurotus ostreatus, Appl Environ Microbiol. 64(10):4028-34. doi: 10.1128/AEM.64.10.4028-4034.1998. PMID: 9758836; PMCID: PMC106595.