Difference between revisions of "Part:BBa K4365022"
Svetlana ia (Talk | contribs) (→Characterization of the pSB1KY in comparison to the p426 shuttle vector) |
|||
| (27 intermediate revisions by one other user not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K4365022 short</partinfo> | <partinfo>BBa_K4365022 short</partinfo> | ||
| − | backbone for | + | The '''pSB1KY-URA3''' is a shuttle plasmid backbone suitable for the iGEM '''RFC1000 assembly''' in ''Saccharomyces cerevisiae'' with ''URA3'' auxotrophy. It was adapted from pSB1K plasmid and optimized to be expressed in ''Escherichia coli cells'' and ''S. cerevisiae'' with ura3 mutations. |
| + | |||
| + | ''URA3'' is a marker gene that restores ODCase activity in yeast. It allows them to grow on media with no uracil or uridine supplemnts. This generates a selection for yeast expressing ''URA3''. | ||
| + | |||
| + | |||
| + | {| class="wikitable" style="margin-left: auto; margin-right: auto; border: none; width:600px;" | ||
| + | |+ Collection of shuttle vector backbones optimized for ''Saccharomyces cerevisiae'' | ||
| + | |- | ||
| + | ! Backbone ID | ||
| + | ! Selection marker | ||
| + | ! Marker sequence source | ||
| + | |- style="text-align:center;" | ||
| + | |[https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365022 BBa_K4365022]||''URA3''||[http://freegenes.github.io/genes/BBF10K_000474.html Open Yeast Collection ScURA3-marker] | ||
| + | |- style="text-align:center;" | ||
| + | |[https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365023 BBa_K4365023]||''TRP1''||[http://freegenes.github.io/genes/BBF10K_000473.html Open Yeast Collection ScTRP1-marker] | ||
| + | |- style="text-align:center;" | ||
| + | |[https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365024 BBa_K4365024]||''HIS3''||[http://freegenes.github.io/genes/BBF10K_000468.html Open Yeast Collection ScHIS3-marker] | ||
| + | |} | ||
<br> | <br> | ||
| Line 11: | Line 28: | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| − | ==== | + | ====Advantages of pSB1KY shuttle vectors==== |
| − | [[File:BBa_K4365022_plasmid_design.png|700px|center|thumb|Figure | + | |
| + | The pSB1KY (Y stands for yeast) is the adapted [https://parts.igem.org/Part:pSB1K04 pSB1K plasmid] made into shuttle plasmids for the iGEM RFC1000 assembly in ''Saccharomyces cerevisiae''. | ||
| + | |||
| + | We modified and improved the pSB1K backbone to create a new series of shuttle vectors for the expression of proteins in S. cerevisiae. These new backbones, called pSB1KY, possess different auxotropy cassettes so that the user can choose the most suitable one for the yeast strain already available in their laboratory. The pSB1KY plasmids have a multicopy yeast origin of replication - the 2µOri - so that multiple copies can be maintained in yeast cells for increased protein production. The pSB1KY relies on the RFC1000 assembly and allows the users to obtain functioning transcriptional units more efficiently. One of the advatages of this new series of backbones is that they are compatible with the new OpenYeast collection parts and, more importantly, can be assembled together without the need of reassmbly the whole plasmid from scratch, as is the case for the assembly system of the OpenYeast Colleciton. | ||
| + | |||
| + | |||
| + | [[File:BBa_K4365022_plasmid_design.png|700px|center|thumb|Figure 1: '''A''' Plasmid map of the pSB1KY-URA3 shuttle plasmid. Its size after removal of the RFP device (BBa_J04550) is ~4.7 kb. '''B''' Plasmid map of the p426 shuttle plasmid. Its size is ~5.6 Kb. Created with Snapgene.]] | ||
<br> | <br> | ||
| − | |||
| − | |||
| − | + | The addition of a yeast origin of replication and an auxotrophy cassette introduced a new function in the original pSB1K plasmid that allows it not only to be expressed in ''Escherichia coli'' cells but also in eukaryotic cells of ''S. cerevisiae''. | |
| − | + | The origin of replication that we have introduced is a multicopy yeast origin of replication, the [https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365002 2µOri], and it allows multiple copies to be maintained in yeast cells for increased protein production. The availability of the various auxotrophy cassettes in pSB1KY plasmid series enhances the accessibility and versatility of the pSB1KY plasmid since users can now choose the pSB1KY that is most suitable for the yeast strain at hand in their laboratory. | |
| − | + | The series of pSB1KY plasmids complements the Open Yeast Collection. It is compatible with Open Yeast Collection genetic parts and it can be assembled without the need to put together the whole plasmid from scratch, as is the case for the assembly system on which the Open Yeast Collection is based. This means that the users of the Open Yeast Collection can choose three plasmids out of the collection, a promoter, a CDS, and a terminator, and can assemble them directly into the pSB1KY that is compatible with their lab’s auxotrophic yeast strain. | |
| + | |||
| + | The pSB1KY plasmids have shown to have great versatility and have found their first users in TU_Dresden22 partners from [https://2022.igem.wiki/wwu-muenster/part-collection WWU_Münster]. | ||
| + | |||
| + | <br> | ||
| + | ====pSB1KY-URA3 assembly==== | ||
| + | |||
| + | Inserts carrying genetic elements for the maintenance, selection, and replication of the plasmids in yeast were introduced into the [https://parts.igem.org/Part:pSB1K04 pSB1K]. These inserts were a multi-copy [https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365002 2μ Ori sequence] and one of the four of the auxotrophy markers from the [https://stanford.freegenes.org/products/open-yeast-toolkit#description Open Yeast Collection]: | ||
| + | * [https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365022 URA3-marker] | ||
| + | * [https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365023 TRP1-marker] | ||
| + | * [https://parts.igem.org/wiki/index.php?title=Part:BBa_K4365024 HIS3-marker] | ||
| + | |||
| + | Three restriction enzymes were selected from the list of non-cutters in the selection markers, in the backbones, and the 2μ Ori: ''BamH''I, ''Spe''I, and ''Not''I. | ||
| + | |||
| + | These restriction sites were added via primers in a PCR reaction using the plasmids from the Open Yeast Collection as templates. We decided to create a level 1 shuttle plasmid with the ''URA3'' cassette first and use it then as a base for the cloning of other auxotrophy markers (''HIS3 and TRP1''). The pSB1K4 was linearized by PCR with the primers iGEM level1 BamHI and iGEM level1 NotI (Figure 2, Table 1). | ||
| + | |||
| + | {| class="wikitable" style="margin-left: auto; margin-right: auto; border: none; width:700px;" | ||
| + | |+ Table 1. Used primer list. | ||
| + | |- | ||
| + | ! Primer name | ||
| + | ! Sequence | ||
| + | ! Target amplicon | ||
| + | |- | ||
| + | |style="text-align:center;"|iGEM URA3 SpeI || TATATAACTAGTAAGGCGGTTTCCTTGAAATTTTTTTGA || style="text-align:center;"| URA3 | ||
| + | |- | ||
| + | |style="text-align:center;"|iGEM URA3 NotI || TATATAGCGGCCGCTCATGGGTAATAACTGATATAATTAAATTGAAGCTC ||style="text-align:center;"| URA3 | ||
| + | |- | ||
| + | |style="text-align:center;"|iGEM 2µ Ori BamHI || TATATAGGATCCGATCCAATATCAAAGGAAATGATAGCATTGAAG ||style="text-align:center;"| 2µ Ori | ||
| + | |- | ||
| + | |style="text-align:center;"|iGEM 2µ Ori SpeI || TATATAACTAGTCATTTCCCCGAAAAGTGCC ||style="text-align:center;"| 2µ Ori | ||
| + | |- | ||
| + | |style="text-align:center;"|iGEM level 1 BamHI || ATAGGATCCGTGAGCGAGGAAGCCTGC ||style="text-align:center;"| level 1 plasmid | ||
| + | |- | ||
| + | |style="text-align:center;"|iGEM level 1 NotI || ATAGCGGCCGCGACTCGCTGCGCTCGG ||style="text-align:center;"| level 1 plasmid | ||
| + | |} | ||
| + | |||
| + | <br> | ||
| + | [[File:BBa_K4365022_linear.png|500px|center|thumb|Figure 2: linearization by PCR of the pSB1k level 1 plasmid and addition of flanking 1 ''BamH''I and ''Not''I restriction sites. Created with Biorender.com.]] | ||
| + | |||
| + | <br>The ''URA3'' cassette, containing the ''URA3'' promoter, the ''URA3'' coding sequence, and the ''URA3'' terminator, was obtained by PCR with the iGEM URA3 SpeI and iGEM URA3 NotI primers using the ScURA3-marker (BBF10K_000474) of the Open Yeast collection as a template. The 2µ Ori was amplified from the widely used p426 shuttle plasmid <ref name="mumberg">Mumberg D, Müller R, Funk M. (1995) Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds, Gene;156(1):119-122. doi:10.1016/0378-1119(95)00037-7</ref> using the iGEM 2µ Ori BamHI and iGEM 2µ Ori SpeI primers. | ||
| + | |||
| + | [[File:BBa_K4365022_insert.png|500px|center|thumb|Figure 3: amplification of the 2µ Ori insert and auxotrophy marker insert form the open yeast collection using PCR. Created with Biorender.com.]] | ||
| + | |||
| + | <br>The amplification of the ''URA3'' cassette and the 2µ Ori was performed with Phusion Flash High-Fidelity (Thermo Scientific), whereas the PCR linearization was carried out with Q5 High-Fidelity DNA Polymerase (NEB). The amplicons were cleaned up (QIAquick PCR Purification Kit, Qiagen) and digested. The pSB1K4 was digested with ''BamH''I and ''Not''I (NEB). The ''URA3'' cassette was digested with ''Spe''I and ''Not''I (NEB). The 2µ Ori was digested with BamHI and SpeI and also dephosphorylated with Antarctic Phosphatase (NEB). After clean-up (QIAquick PCR Purification Kit, Qiagen), the parts were combined in a 1:3:3 ratio and ligated at room temperature for 1 hour using the HI T4 ligase (NEB) (Figure 4). | ||
| + | |||
| + | [[File:BBa_K4365022_full_scheme.png|500px|center|thumb|Figure 4: combination of linearized pSB1k backbone, 2µ Ori insert and auxotrophy marker to create the pSB1KY level 1 shuttle plasmid. Created with Biorender.com.]] | ||
| + | |||
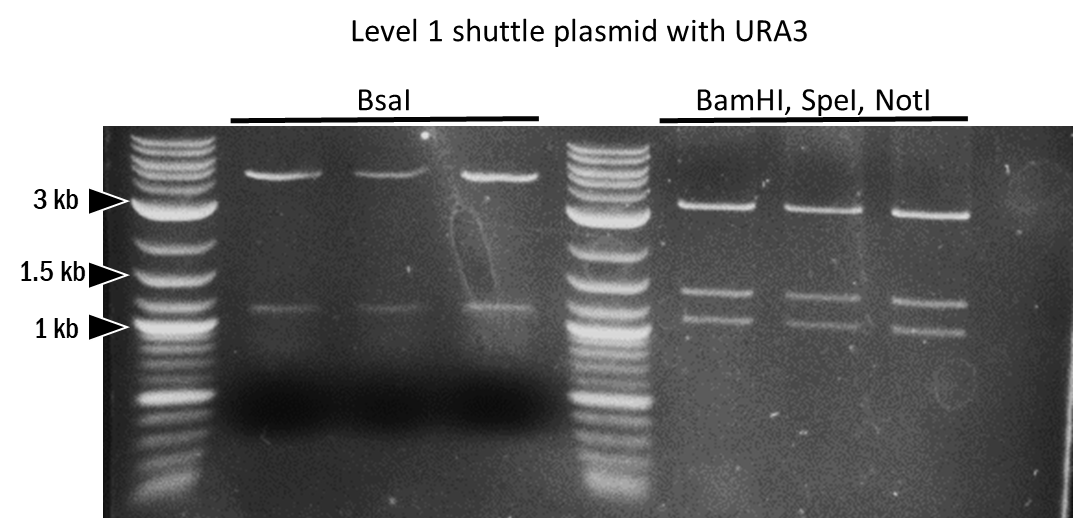
| + | <br>The ligation was transformed, plated on Agar Kanamycin plates, and incubated at 37°C overnight. The next day, red colonies were picked and grown overnight in LB Kanamycin. After isolation of the plasmid ((ZR Plasmid Miniprep - Classic, Zymo Research), the plasmids were digested with ''BamH''I, ''Spe''I (''Bcu''I), and ''Not''I (FD Thermo Firsher), and run on a 1% agarose gel (Figure 6). A 3 kb band, a 1.4 kb band, and the 1 kb band corresponded respectively to the pSB1K backbone, the 2µ Ori insert, and the ''URA3'' cassette insert. Three plasmids were sent for Sanger sequencing (Microsynth). The sequencing results confirmed the ligation of the two inserts into the pSB1K backbone was successful. To test whether the ''Bsa''I sites were still unaltered and available for the following cloning steps, a test digest was conducted using ''Bsa''I (NEB)(Figure 6). | ||
| + | |||
| + | [[File:BBa_K4365022_test_digest1.png|500px|center|thumb|Figure 5: Test digest for the identification of the integration of the 2µ Ori insert and the ''URA3'' cassette into the pSB1K plasmid.]] [[File:BBa_K4365022_test_digest2.png|500px|center|thumb|Figure 6: Test digest to confirm the correct assembly of the pSB1KY and the maintenance of the available ''Bsa''I sites for the subsequent cloning steps.]] | ||
| + | <br> | ||
| + | |||
| + | ====Characterization of the pSB1KY in comparison to the p426 shuttle vector==== | ||
| + | |||
| + | To understand whether the new functionalities introduced into the pSB1K were suitable for subsequent experiments, we compared the growth curves of the pSB1KY-transformed yeast cells and p426-transformed ones expressing turboRFP. Two main cultures were adjusted to 1 OD in 20 ml of MV medium in flasks and, after 12 hr, their growth rates were monitored using a spectrophotometer. | ||
| + | From the plots of the growth rates (Figure 7) we were able to observe that the new pSB1KY performs just as well as the widely used p426 shuttle plasmid <ref name="mumberg" />. | ||
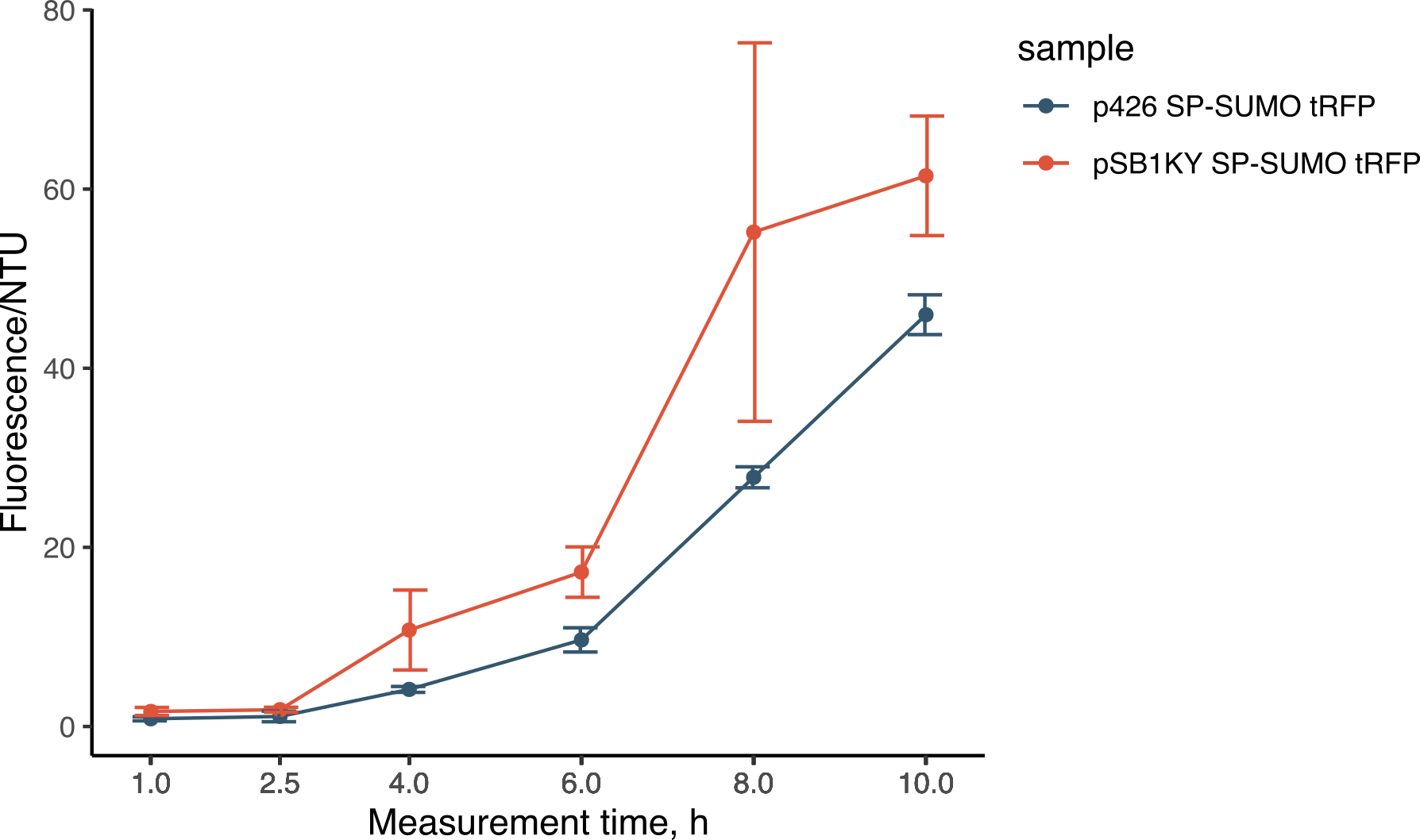
| + | During the experiments for the creation of the SP-SUMO tag for protein production in yeast (read more about it here), we were once again able to compare the two plasmids. Both the p426 and the new pSB1KY were used to express the SP-SUMO device aminoterminally fused to the turboRFP fluorescent protein. This construct allowed the protein to be secreted in the medium and we were able to measure the development of fluorescence. The fluorescence intensity in the medium normalized over growth (expressed in nephelometric turbidity units) (Figure 8) showed that the pSB1KY is able to produce and secrete larger quantities of turboRFP. This was observed in two other experiments (data not shown) and seems to indicate that the pSB1KY allows for higher protein production. | ||
| + | <div><ul> | ||
| + | <li style="display: inline-block;"> [[File:BBa_K4365022_growth_rate.png|thumb|center|350px|Figure 7: growth rates of yeast cells transformed with the new pSB1KY and the p426 shuttle plasmids.]] </li> | ||
| + | <li style="display: inline-block;"> [[File:BBa_K4365022_fluo.png|400px|thumb|center|Figure 8: fluorescence development normalized over nephelometric turbidity units of the expression and secretion of SP-SUMO from the p426 and the new pSB1KY.]] </li> | ||
| + | </ul></div> | ||
| + | ===References=== | ||
| + | <references /> | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Latest revision as of 12:23, 12 October 2022
pSB1KY-URA3
The pSB1KY-URA3 is a shuttle plasmid backbone suitable for the iGEM RFC1000 assembly in Saccharomyces cerevisiae with URA3 auxotrophy. It was adapted from pSB1K plasmid and optimized to be expressed in Escherichia coli cells and S. cerevisiae with ura3 mutations.
URA3 is a marker gene that restores ODCase activity in yeast. It allows them to grow on media with no uracil or uridine supplemnts. This generates a selection for yeast expressing URA3.
| Backbone ID | Selection marker | Marker sequence source |
|---|---|---|
| BBa_K4365022 | URA3 | [http://freegenes.github.io/genes/BBF10K_000474.html Open Yeast Collection ScURA3-marker] |
| BBa_K4365023 | TRP1 | [http://freegenes.github.io/genes/BBF10K_000473.html Open Yeast Collection ScTRP1-marker] |
| BBa_K4365024 | HIS3 | [http://freegenes.github.io/genes/BBF10K_000468.html Open Yeast Collection ScHIS3-marker] |
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Plasmid lacks a prefix.
Plasmid lacks a suffix.
Illegal EcoRI site found at 4632
Illegal XbaI site found at 473
Illegal SpeI site found at 1481
Illegal PstI site found at 12 - 12INCOMPATIBLE WITH RFC[12]Plasmid lacks a prefix.
Plasmid lacks a suffix.
Illegal EcoRI site found at 4632
Illegal SpeI site found at 1481
Illegal PstI site found at 12
Illegal NotI site found at 2554 - 21INCOMPATIBLE WITH RFC[21]Plasmid lacks a prefix.
Plasmid lacks a suffix.
Illegal EcoRI site found at 4632
Illegal BamHI site found at 108
Illegal XhoI site found at 3482
Illegal XhoI site found at 4508 - 23INCOMPATIBLE WITH RFC[23]Plasmid lacks a prefix.
Plasmid lacks a suffix.
Illegal EcoRI site found at 4632
Illegal XbaI site found at 473
Illegal SpeI site found at 1481
Illegal PstI site found at 12 - 25INCOMPATIBLE WITH RFC[25]Plasmid lacks a prefix.
Plasmid lacks a suffix.
Illegal EcoRI site found at 4632
Illegal XbaI site found at 473
Illegal SpeI site found at 1481
Illegal PstI site found at 12 - 1000INCOMPATIBLE WITH RFC[1000]Plasmid lacks a prefix.
Plasmid lacks a suffix.
Illegal SapI site found at 4638
Illegal SapI.rc site found at 5
Usage and Biology
Advantages of pSB1KY shuttle vectors
The pSB1KY (Y stands for yeast) is the adapted pSB1K plasmid made into shuttle plasmids for the iGEM RFC1000 assembly in Saccharomyces cerevisiae.
We modified and improved the pSB1K backbone to create a new series of shuttle vectors for the expression of proteins in S. cerevisiae. These new backbones, called pSB1KY, possess different auxotropy cassettes so that the user can choose the most suitable one for the yeast strain already available in their laboratory. The pSB1KY plasmids have a multicopy yeast origin of replication - the 2µOri - so that multiple copies can be maintained in yeast cells for increased protein production. The pSB1KY relies on the RFC1000 assembly and allows the users to obtain functioning transcriptional units more efficiently. One of the advatages of this new series of backbones is that they are compatible with the new OpenYeast collection parts and, more importantly, can be assembled together without the need of reassmbly the whole plasmid from scratch, as is the case for the assembly system of the OpenYeast Colleciton.
The addition of a yeast origin of replication and an auxotrophy cassette introduced a new function in the original pSB1K plasmid that allows it not only to be expressed in Escherichia coli cells but also in eukaryotic cells of S. cerevisiae.
The origin of replication that we have introduced is a multicopy yeast origin of replication, the 2µOri, and it allows multiple copies to be maintained in yeast cells for increased protein production. The availability of the various auxotrophy cassettes in pSB1KY plasmid series enhances the accessibility and versatility of the pSB1KY plasmid since users can now choose the pSB1KY that is most suitable for the yeast strain at hand in their laboratory.
The series of pSB1KY plasmids complements the Open Yeast Collection. It is compatible with Open Yeast Collection genetic parts and it can be assembled without the need to put together the whole plasmid from scratch, as is the case for the assembly system on which the Open Yeast Collection is based. This means that the users of the Open Yeast Collection can choose three plasmids out of the collection, a promoter, a CDS, and a terminator, and can assemble them directly into the pSB1KY that is compatible with their lab’s auxotrophic yeast strain.
The pSB1KY plasmids have shown to have great versatility and have found their first users in TU_Dresden22 partners from WWU_Münster.
pSB1KY-URA3 assembly
Inserts carrying genetic elements for the maintenance, selection, and replication of the plasmids in yeast were introduced into the pSB1K. These inserts were a multi-copy 2μ Ori sequence and one of the four of the auxotrophy markers from the Open Yeast Collection:
Three restriction enzymes were selected from the list of non-cutters in the selection markers, in the backbones, and the 2μ Ori: BamHI, SpeI, and NotI.
These restriction sites were added via primers in a PCR reaction using the plasmids from the Open Yeast Collection as templates. We decided to create a level 1 shuttle plasmid with the URA3 cassette first and use it then as a base for the cloning of other auxotrophy markers (HIS3 and TRP1). The pSB1K4 was linearized by PCR with the primers iGEM level1 BamHI and iGEM level1 NotI (Figure 2, Table 1).
| Primer name | Sequence | Target amplicon |
|---|---|---|
| iGEM URA3 SpeI | TATATAACTAGTAAGGCGGTTTCCTTGAAATTTTTTTGA | URA3 |
| iGEM URA3 NotI | TATATAGCGGCCGCTCATGGGTAATAACTGATATAATTAAATTGAAGCTC | URA3 |
| iGEM 2µ Ori BamHI | TATATAGGATCCGATCCAATATCAAAGGAAATGATAGCATTGAAG | 2µ Ori |
| iGEM 2µ Ori SpeI | TATATAACTAGTCATTTCCCCGAAAAGTGCC | 2µ Ori |
| iGEM level 1 BamHI | ATAGGATCCGTGAGCGAGGAAGCCTGC | level 1 plasmid |
| iGEM level 1 NotI | ATAGCGGCCGCGACTCGCTGCGCTCGG | level 1 plasmid |
The URA3 cassette, containing the URA3 promoter, the URA3 coding sequence, and the URA3 terminator, was obtained by PCR with the iGEM URA3 SpeI and iGEM URA3 NotI primers using the ScURA3-marker (BBF10K_000474) of the Open Yeast collection as a template. The 2µ Ori was amplified from the widely used p426 shuttle plasmid [1] using the iGEM 2µ Ori BamHI and iGEM 2µ Ori SpeI primers.
The amplification of the URA3 cassette and the 2µ Ori was performed with Phusion Flash High-Fidelity (Thermo Scientific), whereas the PCR linearization was carried out with Q5 High-Fidelity DNA Polymerase (NEB). The amplicons were cleaned up (QIAquick PCR Purification Kit, Qiagen) and digested. The pSB1K4 was digested with BamHI and NotI (NEB). The URA3 cassette was digested with SpeI and NotI (NEB). The 2µ Ori was digested with BamHI and SpeI and also dephosphorylated with Antarctic Phosphatase (NEB). After clean-up (QIAquick PCR Purification Kit, Qiagen), the parts were combined in a 1:3:3 ratio and ligated at room temperature for 1 hour using the HI T4 ligase (NEB) (Figure 4).
The ligation was transformed, plated on Agar Kanamycin plates, and incubated at 37°C overnight. The next day, red colonies were picked and grown overnight in LB Kanamycin. After isolation of the plasmid ((ZR Plasmid Miniprep - Classic, Zymo Research), the plasmids were digested with BamHI, SpeI (BcuI), and NotI (FD Thermo Firsher), and run on a 1% agarose gel (Figure 6). A 3 kb band, a 1.4 kb band, and the 1 kb band corresponded respectively to the pSB1K backbone, the 2µ Ori insert, and the URA3 cassette insert. Three plasmids were sent for Sanger sequencing (Microsynth). The sequencing results confirmed the ligation of the two inserts into the pSB1K backbone was successful. To test whether the BsaI sites were still unaltered and available for the following cloning steps, a test digest was conducted using BsaI (NEB)(Figure 6).
Characterization of the pSB1KY in comparison to the p426 shuttle vector
To understand whether the new functionalities introduced into the pSB1K were suitable for subsequent experiments, we compared the growth curves of the pSB1KY-transformed yeast cells and p426-transformed ones expressing turboRFP. Two main cultures were adjusted to 1 OD in 20 ml of MV medium in flasks and, after 12 hr, their growth rates were monitored using a spectrophotometer.
From the plots of the growth rates (Figure 7) we were able to observe that the new pSB1KY performs just as well as the widely used p426 shuttle plasmid [1].
During the experiments for the creation of the SP-SUMO tag for protein production in yeast (read more about it here), we were once again able to compare the two plasmids. Both the p426 and the new pSB1KY were used to express the SP-SUMO device aminoterminally fused to the turboRFP fluorescent protein. This construct allowed the protein to be secreted in the medium and we were able to measure the development of fluorescence. The fluorescence intensity in the medium normalized over growth (expressed in nephelometric turbidity units) (Figure 8) showed that the pSB1KY is able to produce and secrete larger quantities of turboRFP. This was observed in two other experiments (data not shown) and seems to indicate that the pSB1KY allows for higher protein production.
References
- ↑ 1.0 1.1 Mumberg D, Müller R, Funk M. (1995) Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds, Gene;156(1):119-122. doi:10.1016/0378-1119(95)00037-7