Difference between revisions of "Part:BBa K4345000"
(→Usage and Biology) |
|||
| (15 intermediate revisions by the same user not shown) | |||
| Line 8: | Line 8: | ||
This part is composed of BBa_K4345007 (NarX), BBa_K4345002 (rigid linker) and BBa_K4345009 (mNeonGreen). | This part is composed of BBa_K4345007 (NarX), BBa_K4345002 (rigid linker) and BBa_K4345009 (mNeonGreen). | ||
| + | |||
| + | |||
| + | ===Sequence and Features=== | ||
| + | <partinfo>BBa_K4345000 SequenceAndFeatures</partinfo> | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
This particular narX protein was derived from ''E. coli''. | This particular narX protein was derived from ''E. coli''. | ||
| − | [[Image:NarX nitrate Cheung&Hendrickson2009.jpeg| | + | |
| + | |||
| + | [[Image:NarX nitrate Cheung&Hendrickson2009.jpeg|500px]] | ||
| + | |||
| + | Image obtained from Cheung & Hendrickson, 2009 | ||
| + | |||
| + | ===References=== | ||
| + | |||
| + | Cavicchioli, R., Schröder, I., Schröder, S., Constanti, M., & Gunsalus, R. P. (1995). The NarX and NarQ Sensor-Transmitter Proteins of Escherichia coli Each Require Two Conserved Histidines for Nitrate-Dependent Signal Transduction to NarL. JOURNAL OF BACTERIOLOGY, 177(9), 2416–2424. | ||
| + | |||
| + | Cheung, J., & Hendrickson, W. A. (2009). Structural Analysis of Ligand Stimulation of the Histidine Kinase NarX. Structure, 17(2), 190–201. https://doi.org/10.1016/J.STR.2008.12.013 | ||
| + | |||
| + | narX sensor histidine kinase NarX [ Escherichia coli str. K-12 substr. MG1655 ]. (2022, September 22). National Library of Medicine - National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/gene/945788 | ||
Latest revision as of 10:29, 8 October 2022
NarX fused to mNeonGreen with a rigid linker
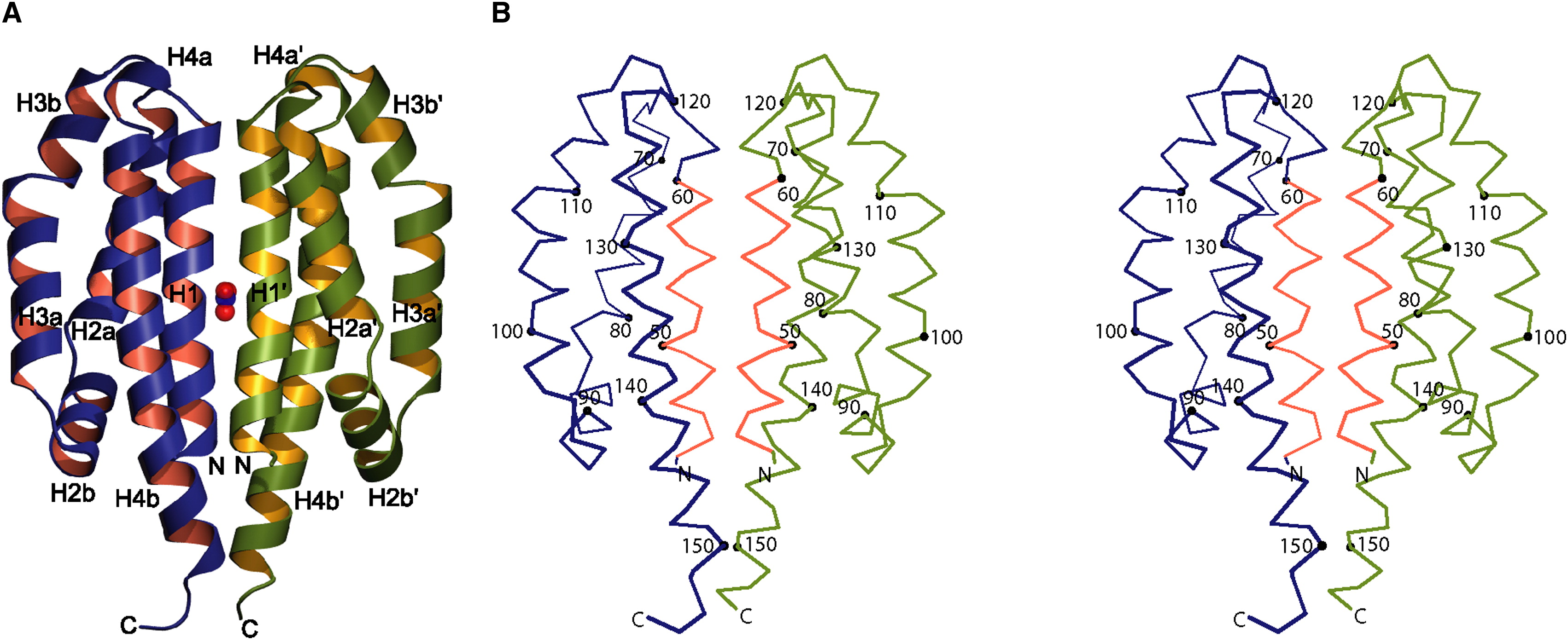
Three proteins constitute a two-component nitrate sensing system in Escherichia coli: NarX, NarL and NarQ. Together they are responsible for the expression of anaerobic respiratory genes. NarX and NarQ are histidine kinases that independently detect the presence of nitrate an transmit the signal to NarL. After autophosphorylation and thus dimerization of NarX or NarQ, NarL is phosphorylated. This enables the activated NarL to bind DNA and induce expression of specific genes. Both NarX and narQ contain two conserved histidine residues that correspond to the autophosphorylation sites of other, homologous, sensor-transmitter proteins (Cavicchioli et al., 1995). Cheung & Hendrickson (2009) elucidated the structure of the histidine kinase in the apo- and holo-state to be a four-helix bundle. To follow the expression of NarX, it is fused to mNeonGreen with a rigid linker.
This part is composed of BBa_K4345007 (NarX), BBa_K4345002 (rigid linker) and BBa_K4345009 (mNeonGreen).
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 659
- 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 659
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 260
- 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 659
- 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 659
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 2236
Usage and Biology
This particular narX protein was derived from E. coli.
Image obtained from Cheung & Hendrickson, 2009
References
Cavicchioli, R., Schröder, I., Schröder, S., Constanti, M., & Gunsalus, R. P. (1995). The NarX and NarQ Sensor-Transmitter Proteins of Escherichia coli Each Require Two Conserved Histidines for Nitrate-Dependent Signal Transduction to NarL. JOURNAL OF BACTERIOLOGY, 177(9), 2416–2424.
Cheung, J., & Hendrickson, W. A. (2009). Structural Analysis of Ligand Stimulation of the Histidine Kinase NarX. Structure, 17(2), 190–201. https://doi.org/10.1016/J.STR.2008.12.013
narX sensor histidine kinase NarX [ Escherichia coli str. K-12 substr. MG1655 ]. (2022, September 22). National Library of Medicine - National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/gene/945788