Difference between revisions of "Part:BBa K4345020"
| (4 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K4345020 short</partinfo> | <partinfo>BBa_K4345020 short</partinfo> | ||
| − | Design of | + | Design of a thermoregulated translation of antitoxin ccdA. Fluorescent protein sfGFP was linked to the antitoxin with a rigid linker for visualisation. |
| Line 10: | Line 10: | ||
<!-- --> | <!-- --> | ||
| − | + | ===Sequence and Features=== | |
<partinfo>BBa_K4345020 SequenceAndFeatures</partinfo> | <partinfo>BBa_K4345020 SequenceAndFeatures</partinfo> | ||
| − | + | ===Usage and Biology=== | |
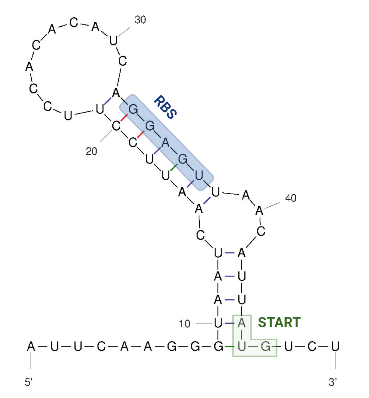
| − | === | + | The figure below shows the secondary structure of the RNA thermometer. |
| − | < | + | |
| − | + | [[Image:Hsp 17 UNAFold Biorender Kortmann et al 2010.png|300px]] | |
| + | |||
| + | Image obtained from Kortmann et al., 2010, edited with biorender and UNAfold. | ||
| + | |||
| + | |||
| + | ===Results=== | ||
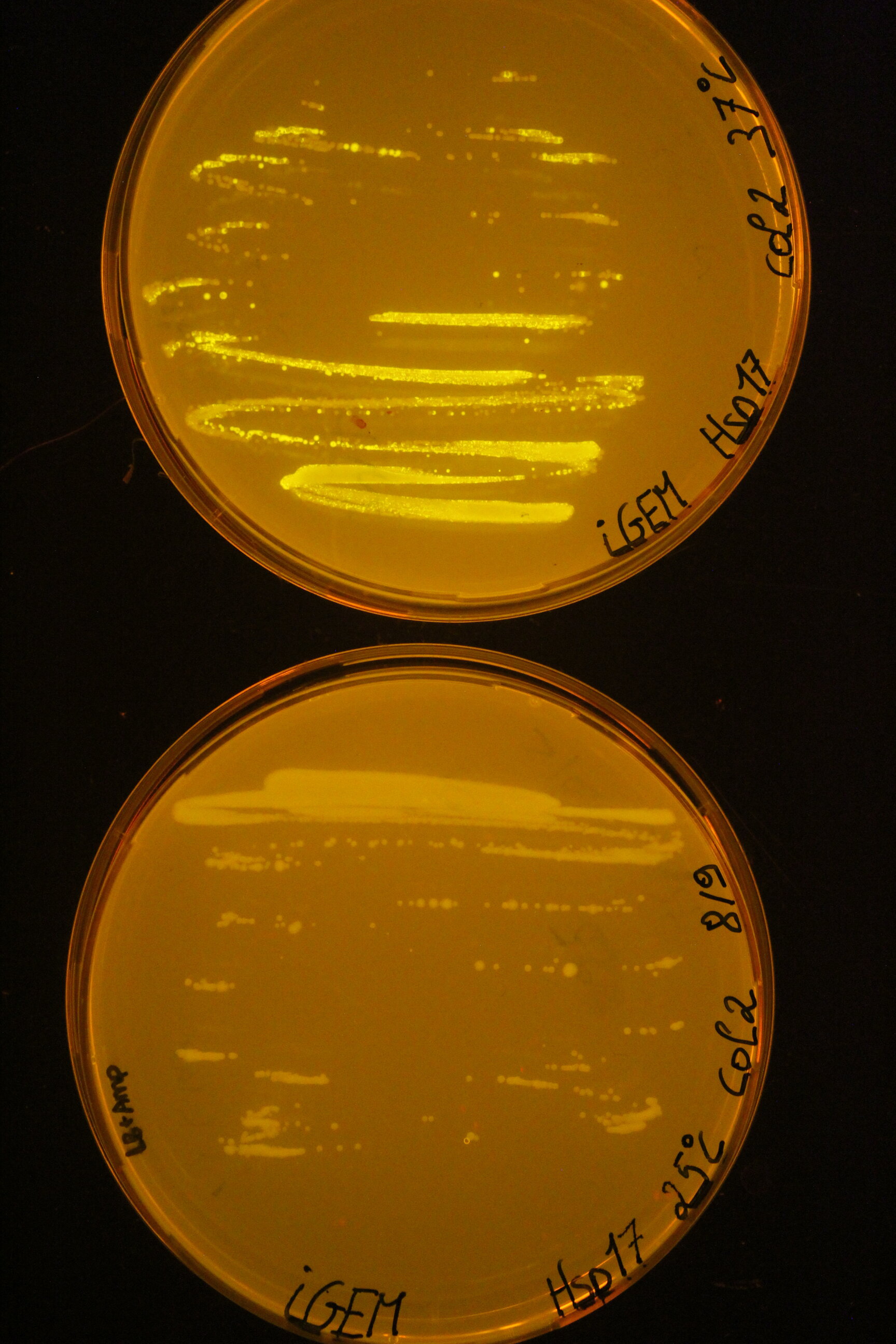
| + | Below you can find an example of the results found after the induction tests of the RNA thermometer. | ||
| + | |||
| + | Two 96 well plates were prepared with 200 µL of the relevant selection medium (LB + 100 µg/mL ampicillin) and the plates were covered with a breathable film. The cultures ''E. coli'' DH5α were diluted 50 times in these well plates and grown at 25°C or 37°C overnight at 180 rpm. The next day, both the OD<sub>600</sub> as the fluorescence intensity were measured with the CLARIOstar (BMG Labtech, Ortenberg, Germany). Blancs (LB) and negative controls (culture with pLO_SNAP plasmid) are subjected to the same protocol. All measurements were performed in triplicate. | ||
| + | To visualise the results for this picture, the successful cultures were grown on plates at the different temperatures. | ||
| + | |||
| + | [[Image:Hsp17 result.jpeg|300px]] | ||
| + | |||
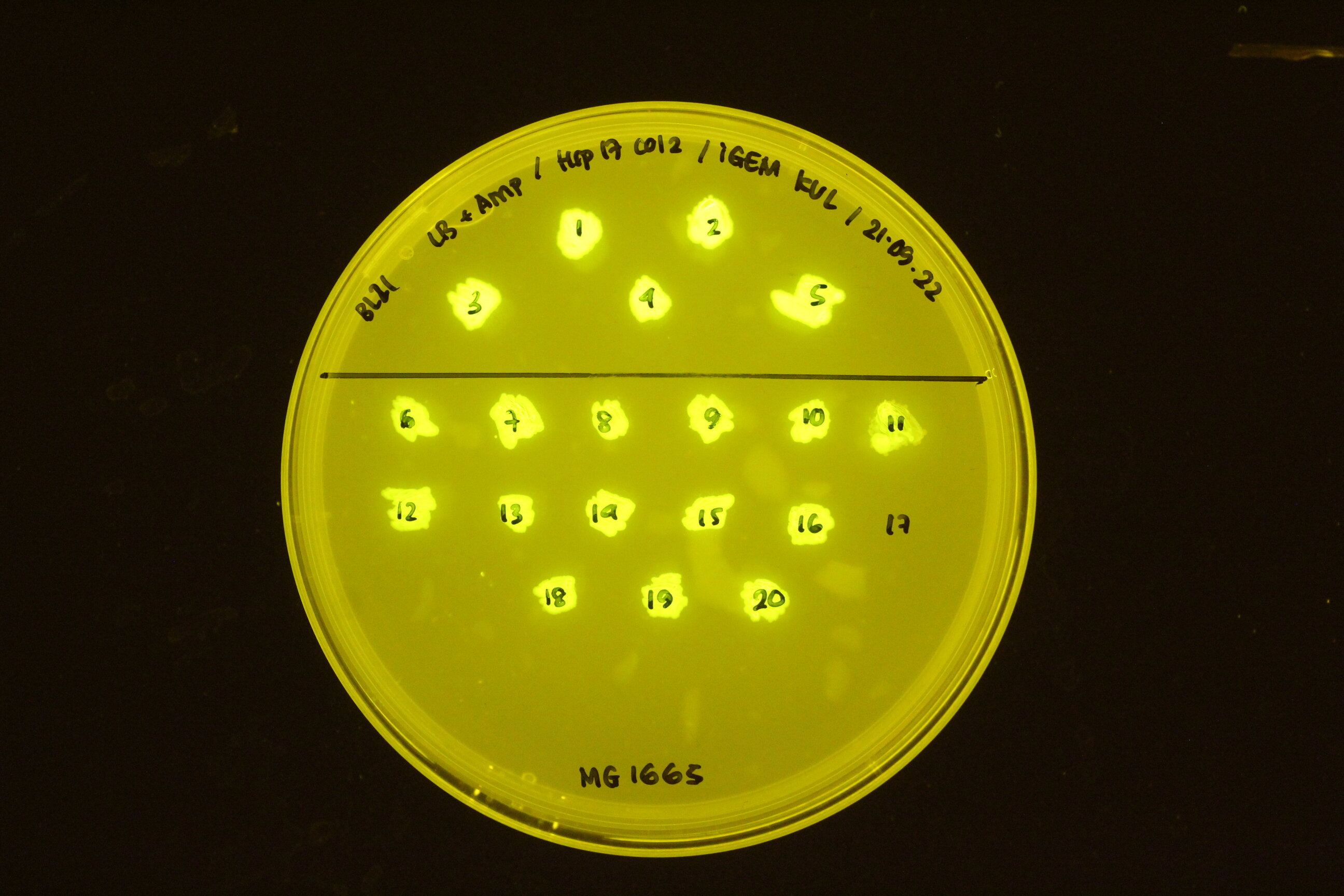
| + | In the figure below, the construct transformed into different cell types: BL21 (top) and K12 M1665 (bottom). | ||
| + | |||
| + | [[Image:Hsp17 in BL21 and K12 MG 1665.jpeg|300px]] | ||
| + | |||
| + | ===References=== | ||
| + | Kortmann, J., Sczodrok, S., Rinnenthal, J., Schwalbe, H., & Narberhaus, F. (2010, December 3). Translation on demand by a simple RNA-based thermosensor. Nucleic Acids Research, 39(7), 2855–2868. https://doi.org/10.1093/nar/gkq1252 | ||
Latest revision as of 15:03, 8 October 2022
5' UTR of hsp17 with ccdA fused to sfGFP
Design of a thermoregulated translation of antitoxin ccdA. Fluorescent protein sfGFP was linked to the antitoxin with a rigid linker for visualisation.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 1149
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 569
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
The figure below shows the secondary structure of the RNA thermometer.
Image obtained from Kortmann et al., 2010, edited with biorender and UNAfold.
Results
Below you can find an example of the results found after the induction tests of the RNA thermometer.
Two 96 well plates were prepared with 200 µL of the relevant selection medium (LB + 100 µg/mL ampicillin) and the plates were covered with a breathable film. The cultures E. coli DH5α were diluted 50 times in these well plates and grown at 25°C or 37°C overnight at 180 rpm. The next day, both the OD600 as the fluorescence intensity were measured with the CLARIOstar (BMG Labtech, Ortenberg, Germany). Blancs (LB) and negative controls (culture with pLO_SNAP plasmid) are subjected to the same protocol. All measurements were performed in triplicate. To visualise the results for this picture, the successful cultures were grown on plates at the different temperatures.
In the figure below, the construct transformed into different cell types: BL21 (top) and K12 M1665 (bottom).
References
Kortmann, J., Sczodrok, S., Rinnenthal, J., Schwalbe, H., & Narberhaus, F. (2010, December 3). Translation on demand by a simple RNA-based thermosensor. Nucleic Acids Research, 39(7), 2855–2868. https://doi.org/10.1093/nar/gkq1252