Difference between revisions of "Part:BBa K4257000"
(→Data cpu_nanjing) |
|||
| (17 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
| − | |||
| − | |||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K4257000 short</partinfo> | <partinfo>BBa_K4257000 short</partinfo> | ||
| − | + | PPK-M is a mutant of E. coli polyphosphate kinase (PPK), which catalyzes the synthesis of polyphosphate (polyP) using cellular ATP as the substrate. Compared to native E. coli PPK that has an alanine and a glutamine residue in position 327 and 328, PPK-M has much more strongly charged glutamate and lysine residues. It has been documented that expression of PPK-M leads to substantially higher levels of polyP accumulation in vivo by disrupting intracellular PPK-repressing interactions, as compared to the situation found with expression of PPK (Rudat et al. 2018). PolyP consists of inorganic phosphate, which is essentially derived from the uptake of exogenous phosphorus by the host cell. For this reason, if enhanced uptake of exogenous phosphorus by the host cell is desired, PPK-M will be a better option. This year, our team wants to develop an engineered E. coli K12 strain that can use phosphite as a raw material to manufacture phosphate, in which process intracellular polyP is the intermediate. Therefore, we picked highly active PPK-M. | |
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 13: | Line 10: | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K4257000 SequenceAndFeatures</partinfo> | <partinfo>BBa_K4257000 SequenceAndFeatures</partinfo> | ||
| + | |||
| + | |||
| + | |||
| + | ===Data:CPU-Nanjing 2022 TEAM=== | ||
| + | The yield of our final product is positively correlated with the amount of intracellular polyP and the cell biomass. | ||
| + | |||
| + | |||
| + | 1. Biomass | ||
| + | |||
| + | We first compared the biomass of two engineered E. coli K12 strains that overexpress PPK and PPK-M using synthetic municipal wastewater (SMW) medium. | ||
| + | [[File:CPU-Nanjing-Parts-PPK-M-11.png|300px|center]] | ||
| + | <center>Figure 1. Maximum optical density of K12/PPK-M grown in synthetic municipal wastewater compared with K12/PPK.</center> | ||
| + | |||
| + | As shown in Figure 1, the biomass of K12/PPK-M is almost identical to that of K12/PPK, indicating that highly active PPK-M would not impose additional metabolic burden upon the host cell. | ||
| + | |||
| + | |||
| + | 2. Intracellular polyP | ||
| + | |||
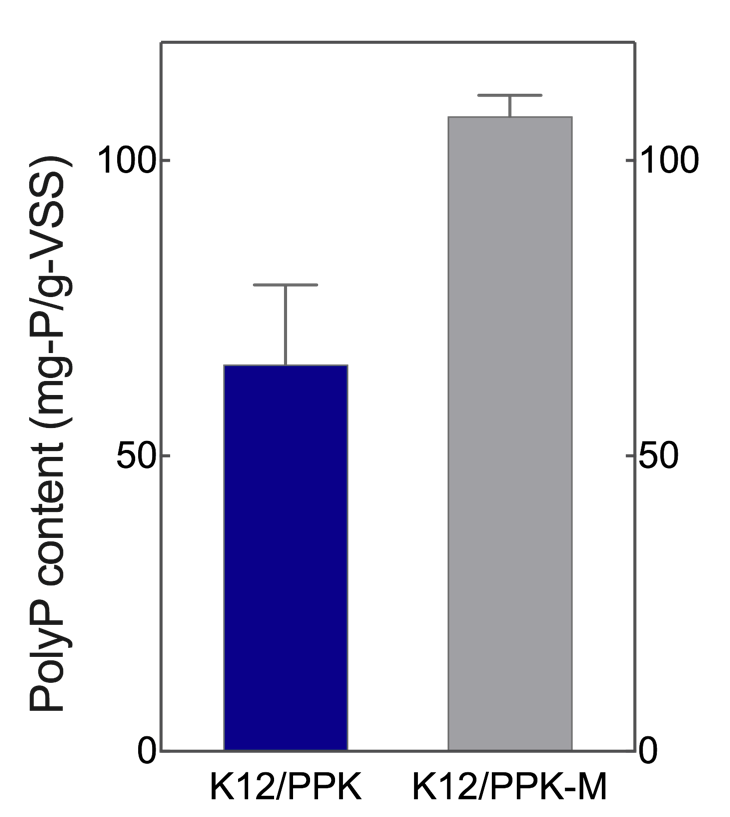
| + | As expected, intracellular polyP assay showed that the amount of polyP produced by K12/PPK-M is significantly higher than that obtained by K12/PPK (Figure. 2). | ||
| + | [[File:CPU-Nanjing-Parts-PPK-M-21.png|300px|center]] | ||
| + | |||
| + | <center>Figure 2. Intracellular polyP content of K12/PPK-M compared with K12/PPK.</center> | ||
| + | |||
| + | |||
| + | 3. Microscopic observation | ||
| + | |||
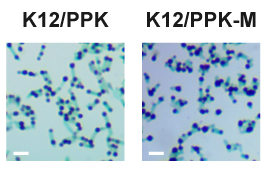
| + | Consistent with quantitative polyP assays, polyP granules observed in K12/PPK-M were significantly larger than those found in K12/PPK (Figure. 3). | ||
| + | [[File:CPU-Nanjing-Parts-PPK-M-311.png|300px|center]] | ||
| + | |||
| + | <center>Figure 3. Light microscopy images of toluidine blue stained cells. Intracellular polyP granules appear blue-purple, whereas cells appear blue-green. Scale bar, 2 μm.</center> | ||
| + | |||
| + | |||
| + | ===References=== | ||
| + | Rudat, A.K., Pokhrel, A., Green, T.J. and Gray, M. (2018) Mutations in Escherichia coli polyphosphate kinase that lead to dramatically increased in vivo polyphosphate levels. Journal of Bacteriology 200(6), e00697-00617. | ||
| + | |||
Latest revision as of 12:50, 11 October 2022
PPK-M
PPK-M is a mutant of E. coli polyphosphate kinase (PPK), which catalyzes the synthesis of polyphosphate (polyP) using cellular ATP as the substrate. Compared to native E. coli PPK that has an alanine and a glutamine residue in position 327 and 328, PPK-M has much more strongly charged glutamate and lysine residues. It has been documented that expression of PPK-M leads to substantially higher levels of polyP accumulation in vivo by disrupting intracellular PPK-repressing interactions, as compared to the situation found with expression of PPK (Rudat et al. 2018). PolyP consists of inorganic phosphate, which is essentially derived from the uptake of exogenous phosphorus by the host cell. For this reason, if enhanced uptake of exogenous phosphorus by the host cell is desired, PPK-M will be a better option. This year, our team wants to develop an engineered E. coli K12 strain that can use phosphite as a raw material to manufacture phosphate, in which process intracellular polyP is the intermediate. Therefore, we picked highly active PPK-M.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Data:CPU-Nanjing 2022 TEAM
The yield of our final product is positively correlated with the amount of intracellular polyP and the cell biomass.
1. Biomass
We first compared the biomass of two engineered E. coli K12 strains that overexpress PPK and PPK-M using synthetic municipal wastewater (SMW) medium.
As shown in Figure 1, the biomass of K12/PPK-M is almost identical to that of K12/PPK, indicating that highly active PPK-M would not impose additional metabolic burden upon the host cell.
2. Intracellular polyP
As expected, intracellular polyP assay showed that the amount of polyP produced by K12/PPK-M is significantly higher than that obtained by K12/PPK (Figure. 2).
3. Microscopic observation
Consistent with quantitative polyP assays, polyP granules observed in K12/PPK-M were significantly larger than those found in K12/PPK (Figure. 3).
References
Rudat, A.K., Pokhrel, A., Green, T.J. and Gray, M. (2018) Mutations in Escherichia coli polyphosphate kinase that lead to dramatically increased in vivo polyphosphate levels. Journal of Bacteriology 200(6), e00697-00617.