Difference between revisions of "Part:BBa K4235000"
| (89 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K4235000 short</partinfo> | <partinfo>BBa_K4235000 short</partinfo> | ||
| − | + | <br>Codon optimized for sf9 cells | |
| − | Usage and Biology | + | |
| + | ===Usage and Biology=== | ||
Protein S is a vitamin K-dependent plasma protein that functions to prevent hypercoagulation of the blood. It serves as a non enzymatic cofactor for activated Protein C and is involved in the inactivation of coagulation factors Va and VIIIa. Protein S exists in two states in plasma, about 40% circulates as a free, functionally active form and the remaining 60% exists in the inactive form bound with C4b-binding protein. Protein S is secreted by hepatocytes, megakaryocytes, endothelial cells, etc. The initial form of secreted protein S is a 676 amino acid precursor protein, which undergoes a cleavage of a signal peptide present at the N-terminal, resulting in the mature 635 amino acid protein. Functionally active Protein S can directly bind to inhibit factor IXa, which activates factor X to Xa. Factor Xa and Va together form the prothrombinase complex responsible for activation of thrombin. Moreover, by acting as a cofactor for activated protein C, protein S promotes the cleavage of Factor VIIIa and Va, inhibiting the coagulation cascades. | Protein S is a vitamin K-dependent plasma protein that functions to prevent hypercoagulation of the blood. It serves as a non enzymatic cofactor for activated Protein C and is involved in the inactivation of coagulation factors Va and VIIIa. Protein S exists in two states in plasma, about 40% circulates as a free, functionally active form and the remaining 60% exists in the inactive form bound with C4b-binding protein. Protein S is secreted by hepatocytes, megakaryocytes, endothelial cells, etc. The initial form of secreted protein S is a 676 amino acid precursor protein, which undergoes a cleavage of a signal peptide present at the N-terminal, resulting in the mature 635 amino acid protein. Functionally active Protein S can directly bind to inhibit factor IXa, which activates factor X to Xa. Factor Xa and Va together form the prothrombinase complex responsible for activation of thrombin. Moreover, by acting as a cofactor for activated protein C, protein S promotes the cleavage of Factor VIIIa and Va, inhibiting the coagulation cascades. | ||
| + | |||
Mutations in this gene (inherited as an autosomal dominant, homozygous or heterozygous fashion) cause non-functional or lower plasma levels of Protein S resulting in a Protein S deficiency. Individuals with Protein S deficiency are at an increased risk of developing abnormal blood clots, specifically in the smaller veins, known as venous thromboembolism. Two most common conditions associated with Protein S deficiency are deep vein thrombosis and pulmonary embolism. Although rare, infants with severe protein S deficiency can develop several blood clots throughout the body, resulting in a life threatening condition known as purpura fulminans. Moreover, severe COVID-19 infections are known to cause a decline in protein S levels, which further contributes to infection severity by causing extensive endothelial dysfunction and lung damage, which is a major cause of COVID-related mortality. | Mutations in this gene (inherited as an autosomal dominant, homozygous or heterozygous fashion) cause non-functional or lower plasma levels of Protein S resulting in a Protein S deficiency. Individuals with Protein S deficiency are at an increased risk of developing abnormal blood clots, specifically in the smaller veins, known as venous thromboembolism. Two most common conditions associated with Protein S deficiency are deep vein thrombosis and pulmonary embolism. Although rare, infants with severe protein S deficiency can develop several blood clots throughout the body, resulting in a life threatening condition known as purpura fulminans. Moreover, severe COVID-19 infections are known to cause a decline in protein S levels, which further contributes to infection severity by causing extensive endothelial dysfunction and lung damage, which is a major cause of COVID-related mortality. | ||
| + | <span class='h3bb'></span> | ||
| − | + | <span class='h3bb'>Sequence and Features</span> | |
| + | <partinfo>BBa_K4235000 SequenceAndFeatures</partinfo> | ||
| + | <!-- --> | ||
| − | + | ===Protein modeling=== | |
| − | + | Bioinformatics tools can be used to model a protein structure if the amino-acid sequence of the protein is known. Computational protein structure prediction relies on principles obtained through techniques including X-ray crystallography, NMR spectroscopy and other physical energy functions to predict, with a certain level of accuracy, the three-dimensional structures of proteins. These methods use various Machine Learning algorithms to develop and predict comprehensive protein structures. We decided to use three methods for modeling our protein, protein S, for which there is not a structure available. | |
| + | Here, we used the Ab-Initio Method to model Protein S structure. | ||
| + | When there is not a known structure of a similar protein, this method can be used to determine the tertiary structure of a protein. This method conducts a conformational search using a designed energy function and generates a number of possible conformations. From these, final models can be selected. | ||
| − | + | The Protein S (PROS1) sequence comprises 676 amino acids. Through literature review, it was found that PROS1 is synthesized as a 676 amino acid precursor protein which is processed to a mature protein of 635 amino acids. | |
| − | + | The 41 amino acid difference between the two accounts for a signaling peptide that is necessary for the expression of the protein. A post-translational modification, specifically a simple singular peptide bond cleavage, results in the mature form of the protein that gets secreted. | |
| + | Therefore, we chose to model both the pre-cleaved PROS1 (676 AA), and the truncated mature PROS1 (635 AA). | ||
| − | |||
| − | |||
| − | |||
| + | '''676 Precursor Protein Sequence'''<br> | ||
| + | MRVLGGRCGALLACLLLVLPVSEANFLSKQQASQVLVRKRRANSLLEETKQGNLERECIEELCNKEEARE<br>VFENDPETDYFYPKYLVCLRSFQTGLFTAARQSTNAYPDLRSCVNAIPDQCSPLPCNEDGYMSCKDGKASFTCTCKPGWQGEKCEFDINECKDPSNIN<br>GGCSQICDNTPGSYHCSCKNGFVMLSNKKDCKDVDECSLKPSICGTAVCKNIPGDFECECPEGYRYNLKSKSCEDIDECSENMCAQLCVNYPGGYTCYCDGKKGFKLAQ<br>DQKSCEVVSVCLPLNLDTKYELLYLAEQFAGVVLYLKFRLPEISRFSAEFDFRTYDSEGVILYAESIDHSAWLLIALRGGKIEVQLKNEHTSKITTGGDVINNGLWNMVSVEELE<br>HSISIKIAKEAVMDINKPGPLFKPENGLLETKVYFAGFPRKVESELIKPINPRLDGCIRSWNLMKQGASGIKEIIQEKQNKHCLVTVEKGSYYPGSGIAQFHIDYNNVSSAEGW<br>HVNVTLNIRPSTGTGVMLALVSGNNTVPFAVSLVDSTSEKSQDILLSVENTVIYRIQALSLCSDQQSHLEFRVNRNNLELSTPLKIETISHEDLQRQLAVLDKAMKAKVAT<br>YLGGLPDVPFSATPVNAFYNGCMEVNINGVQLDLDEAISKHNDIRAHSCPSVWKKTKNS | ||
| − | < | + | '''635 Mature Protein Sequence'''<br> |
| − | === | + | ANSLLEETKQGNLERECIEELCNKEEAREVFENDPETDYFYPKYLVCLRSFQTGLFTAARQSTNAYPDLR<br>SCVNAIPDQCSPLPCNEDGYMSCKDGKASFTCTCKPGWQGEKCEFDINECKDPSNINGGCSQICDNTPGSYHCSCKNGFVMLSNKKDCKDVDECSLKPSICGTAVCKNI<br>PGDFECECPEGYRYNLKSKSCEDIDECSENMCAQLCVNYPGGYTCYCDGKKGFKLAQDQKSCEVVSVCLPLNLDTKYELLYLAEQFAGVVLYLKFRLPEISRFSAEFDF<br>RTYDSEGVILYAESIDHSAWLLIALRGGKIEVQLKNEHTSKITTGGDVINNGLWNMVSVEELEHSISIKIAKEAVMDINKPGPLFKPENGLLETKVYFAGFPRKVESEL<br>IKPINPRLDGCIRSWNLMKQGASGIKEIIQEKQNKHCLVTVEKGSYYPGSGIAQFHIDYNNVSSAEGWHVNVTLNIRPSTGTGVMLALVSGNNTVPFAVSLVDSTSEKS<br>QDILLSVENTVIYRIQALSLCSDQQSHLEFRVNRNNLELSTPLKIETISHEDLQRQLAVLDKAMKAKVATYLGGLPDVPFSATPVNAFYNGCMEVNINGVQLDLDEAIS<br>KHNDIRAHSCPSVWKKTKNS |
| − | < | + | |
| − | < | + | <h5>(1.)676 Precursor Protein Sequence model:</h5><br> |
| + | |||
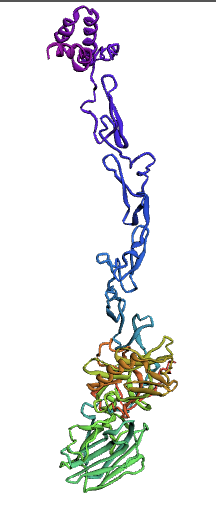
| + | [[Image:676_AA.jpg|400px|thumb|center|'''Figure 1:'''676 Precursor Protein S structure model 5. This structure was modeled using the Robetta serverdeveloped by the Baker lab at the University of Washington.It relies on the Rosetta macromolecular modeling suite developed by the Rosetta Commons.]] | ||
| + | |||
| + | <h5>(2.)635 Precursor Protein Sequence model:</h5><br> | ||
| + | |||
| + | [[Image:635_AA.jpg|400px|thumb|center|'''Figure 2:''' 635 Mature Protein S structure model 3. This structure was modeled using the Robetta serverdeveloped by the Baker lab at the University of Washington.It relies on the Rosetta macromolecular modeling suite developed by the Rosetta Commons. ]] | ||
| + | |||
| + | <h4> Ramachandran plots </h4> | ||
| + | |||
| + | The Ramachandran plot shows the statistical distribution of the combinations of the backbone dihedral angles ϕ and ψ. It gives information about the energetically allowed and disallowed regions in the protein. Having a lower number of residues in the disallowed regions and a high number of residues in the allowed energy regions indicates a good protein structure. We measured this parameter across all models obtained from the ab-initio method. The Ramachandran plots have been generated using MolProbity. It is most complete for crystal structure of proteins and acts as an active validation tool that produces coordinates, graphics and numerical evaluations. Higher weightage was given to the Ramachandran Plot values over the RMSD values. | ||
| + | |||
| + | <h5>(3.)Ramachandran Plot for the 676 Precursor Protein Sequence:</h5> | ||
| + | |||
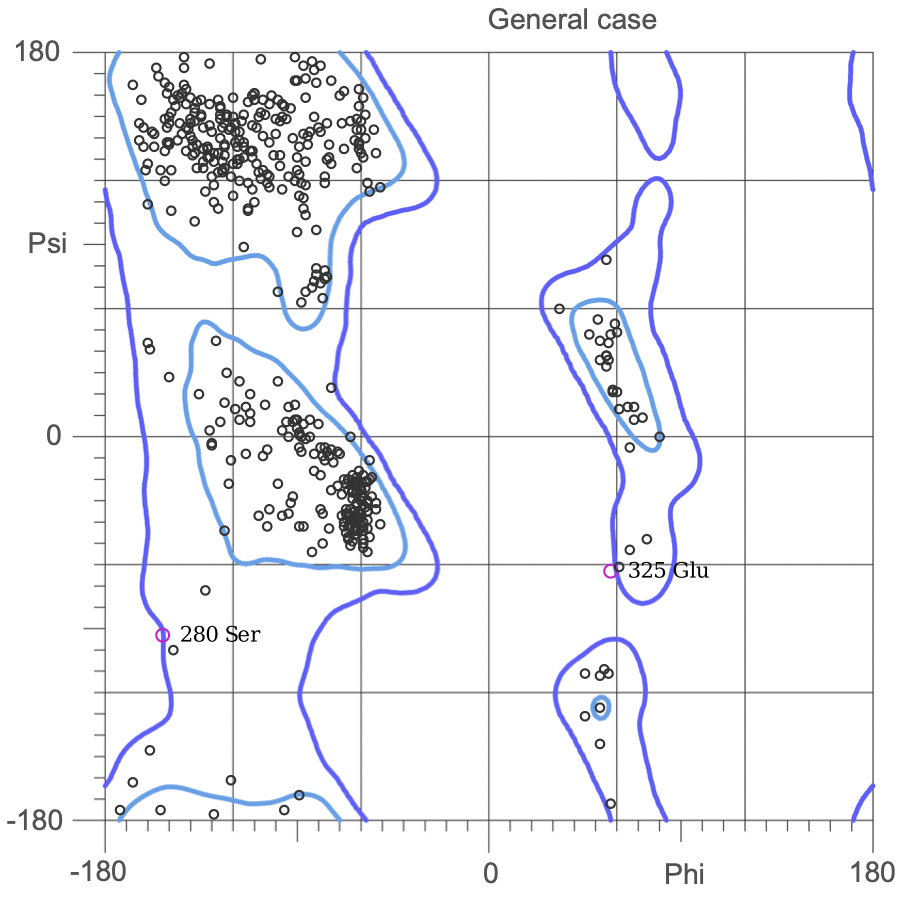
| + | [[Image:676_RP.jpg|400px|thumb|center|'''Figure 3:''' Ramachandran plot (general case) of the 676 AA sequence Ab-initio model 5. As can be seen, 93.6% (631/674) of all residues are in favored (98%) regions. | ||
| + | |||
| + | 99.1% (668/674) of all residues are in allowed (>99.8%) regions. | ||
| + | |||
| + | There were 6 outliers. | ||
| + | ]] | ||
| + | |||
| + | |||
| + | <h5>(4.)Ramachandran plot for the 635 Precursor Protein Sequence:</h5> | ||
| + | |||
| + | [[Image:635_RP.jpg|300px|thumb|center|'''Figure 2:''' Ramachandran plot (general case) of the 635 AA sequence Ab-initio model 3. As can be seen, 94.0% (595/633) of all residues are in favored (98%) regions. | ||
| + | |||
| + | 99.2% (628/633) of all residues are in allowed (>99.8%) regions. | ||
| + | |||
| + | There were 5 outliers. | ||
| + | ]] | ||
| + | |||
| + | '''Conclusion'''<br> | ||
| + | Through protein modeling, we were better able to understand the behavior of protein S, allowing us to understand how cells function and how the misfolded protein can cause disease. This data proved useful in allowing us to understand how the protein structure can change with different genetic mutations, and how this contributes to type I, type II, and type III protein S deficiency.<br> | ||
| + | The protein modeling also gave us better insight into possible mutations that may arise when trying to express a recombinant version of the protein, and what the possible variations of our product might be from natural, functional protein S. Overall, generating possible structures provided us with a greater level of understanding of how protein S works, allowing us to create hypotheses about how to affect, control, and modify it. Even in the future, beyond iGEM, knowing the protein’s structure can also allow us to possibly design site-directed mutations with the intent of changing the protein function. We also plan on looking into the interaction between protein S and protein C, its binding partner, and using protein modeling to help predict their activity. This modeling provides an important first step that offers invaluable insight into our protein structure, function, and molecular dynamics. | ||
| + | |||
| + | ===Mathematical modeling=== | ||
| + | |||
| + | Many biological projects require models that can accurately represent and predict multicomponent, temporally evolving, dynamic systems. Differential equation models are often used for this purpose. This method models the interaction of molecules in the form of rate equations. The system is represented by a system of ordinary differential equations (ODEs), which quantify the interaction between different molecules (i.e. DNA, mRNA or protein), by using the law of mass action. These equations include terms relating to the binding of transcription factors and RNA polymerase to DNA, interactions between transcription factors, mRNA translation rate, and mRNA and protein degradation rates (Ay and Arnosti 2011), for example. In order to accomplish this, knowledge about the system components and structure is required. | ||
| + | |||
| + | We used MATLAB simulations to model our genetic circuits. We used a system of ODEs derived for both constitutive and regulatory genetic circuits(PPBP-Polyhedrin promoter interactions) for our '''SF9''' expression system. We were able to input our system of ODE’s into MATLAB to predict our steady state concentrations prior to starting wet lab in order to determine the process that would yield more favorable results. Those plots can be found below: | ||
| + | |||
| + | '''SF9 constitutive gene circuit model:''' | ||
| + | |||
| + | <h5>(1.) Protein vs time:</h5><br> | ||
| + | [[Image:SF9_const_protein.jpg|400px|thumb|center|'''Figure 1:'''Plot showing Protein production over time ]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| + | <h5>(2.) mRNA vs time:</h5><br> | ||
| + | [[Image:SF9_const_mrna.jpg|400px|thumb|center|'''Figure 2:''' Plot showing mRNA production over time ]] | ||
| + | <br> | ||
| + | |||
| + | |||
| + | <h5>(3.) Effects of altering DNA concentration on protein production:</h5><br> | ||
| + | [[Image:DNA_alter_sf9.jpg|500px|thumb|center|'''Figure 3:''' Plot showing effects of altering initial DNA concentrations on protein production ]] | ||
| + | <br> | ||
| + | |||
| + | |||
| + | '''SF9 Regulatory gene circuit model:'''<br> | ||
| + | |||
| + | The majority of recombinant protein expression is driven by the most powerful baculovirus promoter, polyhedrin, which is active in the late and very late stages of infection. Several studies have been done to study the mechanism of the polyhedrin promoter to better characterize its transcription activity. A host secreted transcription factor, polyhedrin promoter binding protein (PPBP) is known to bind a specific sequence on the polyhedrin promoter with extremely high affinity and specificity and plays a major role in the level of transcription through the polyhedrin promoter. It is established that the PPBP binds to the minor groove of DNA, interacting with the polyhedrin promoter sequence and forming a complex. Further studying the PPBP-DNA interactions and manipulating the concentration of PPBP in host SF9 cells can have a significant impact on the yield of recombinant proteins. | ||
| + | |||
| + | Here, we modeled the interaction between the PPBP and the polyhedrin promoter and its effect on the rate of production of mRNA and the resulting protein S. We were unable to find an established literature value for the concentration/number of molecules of the PPBP in SF9 cells, therefore, we estimated the concentration of PPBP based on some commonly found transcription factors in Drosophila Melanogaster. The plots of MATLAB simulations are listed below: | ||
| + | |||
| + | <h5>(1.) mRNA vs protein S over time:</h5><br> | ||
| + | [[Image:SF9_3D.jpg|500px|thumb|center|'''Figure 4:''' 3D plot showing protein S as a function of mRNA and time ]] | ||
| + | <br> | ||
| + | |||
| + | <h5>(2.) mRNA vs time:</h5><br> | ||
| + | [[Image:SF9_reg_mrna.jpg|400px|thumb|center|'''Figure 5:''' PLot showing mRNA production over time ]] | ||
| + | <br> | ||
| + | |||
| + | |||
| + | <h5>(3.) Protein vs time:</h5><br> | ||
| + | [[Image:SF9_reg_protein.jpg|400px|thumb|center|'''Figure 6:''' Plot showing protein S production over time ]] | ||
| + | <br> | ||
| + | |||
| + | Information on parameter estimation, rate constants and analysis can be found on our model wiki page.<br> | ||
| + | https://2022.igem.wiki/stony-brook/model | ||
| + | |||
| + | Summary: | ||
| + | Modeling the sf9 regulatory gene circuit vs the sf9 constitutive gene circuit provides insight into the role of the Polyhedrin promoter binding protein and its effect on driving robust expression through the polyhedrin promoter. This is evident by the difference in the final steady-state protein values plotted by the regulatory vs the constitutive model. | ||
| + | However, the exact dynamics of PPBP and polyhedrin promoter interactions are not well understood, it was difficult for us to find and approximate the literature values for initial steady state concentrations. Future models and experiments could seek to gain a better understanding of these values in order to make our simulation more accurate and improve our model. | ||
Latest revision as of 23:56, 13 October 2022
Human Protein S Gene (PROS1)
Codon optimized for sf9 cells
Usage and Biology
Protein S is a vitamin K-dependent plasma protein that functions to prevent hypercoagulation of the blood. It serves as a non enzymatic cofactor for activated Protein C and is involved in the inactivation of coagulation factors Va and VIIIa. Protein S exists in two states in plasma, about 40% circulates as a free, functionally active form and the remaining 60% exists in the inactive form bound with C4b-binding protein. Protein S is secreted by hepatocytes, megakaryocytes, endothelial cells, etc. The initial form of secreted protein S is a 676 amino acid precursor protein, which undergoes a cleavage of a signal peptide present at the N-terminal, resulting in the mature 635 amino acid protein. Functionally active Protein S can directly bind to inhibit factor IXa, which activates factor X to Xa. Factor Xa and Va together form the prothrombinase complex responsible for activation of thrombin. Moreover, by acting as a cofactor for activated protein C, protein S promotes the cleavage of Factor VIIIa and Va, inhibiting the coagulation cascades.
Mutations in this gene (inherited as an autosomal dominant, homozygous or heterozygous fashion) cause non-functional or lower plasma levels of Protein S resulting in a Protein S deficiency. Individuals with Protein S deficiency are at an increased risk of developing abnormal blood clots, specifically in the smaller veins, known as venous thromboembolism. Two most common conditions associated with Protein S deficiency are deep vein thrombosis and pulmonary embolism. Although rare, infants with severe protein S deficiency can develop several blood clots throughout the body, resulting in a life threatening condition known as purpura fulminans. Moreover, severe COVID-19 infections are known to cause a decline in protein S levels, which further contributes to infection severity by causing extensive endothelial dysfunction and lung damage, which is a major cause of COVID-related mortality.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal EcoRI site found at 2028
Illegal XbaI site found at 1341 - 12INCOMPATIBLE WITH RFC[12]Illegal EcoRI site found at 2028
- 21INCOMPATIBLE WITH RFC[21]Illegal EcoRI site found at 2028
- 23INCOMPATIBLE WITH RFC[23]Illegal EcoRI site found at 2028
Illegal XbaI site found at 1341 - 25INCOMPATIBLE WITH RFC[25]Illegal EcoRI site found at 2028
Illegal XbaI site found at 1341 - 1000COMPATIBLE WITH RFC[1000]
Protein modeling
Bioinformatics tools can be used to model a protein structure if the amino-acid sequence of the protein is known. Computational protein structure prediction relies on principles obtained through techniques including X-ray crystallography, NMR spectroscopy and other physical energy functions to predict, with a certain level of accuracy, the three-dimensional structures of proteins. These methods use various Machine Learning algorithms to develop and predict comprehensive protein structures. We decided to use three methods for modeling our protein, protein S, for which there is not a structure available.
Here, we used the Ab-Initio Method to model Protein S structure. When there is not a known structure of a similar protein, this method can be used to determine the tertiary structure of a protein. This method conducts a conformational search using a designed energy function and generates a number of possible conformations. From these, final models can be selected.
The Protein S (PROS1) sequence comprises 676 amino acids. Through literature review, it was found that PROS1 is synthesized as a 676 amino acid precursor protein which is processed to a mature protein of 635 amino acids. The 41 amino acid difference between the two accounts for a signaling peptide that is necessary for the expression of the protein. A post-translational modification, specifically a simple singular peptide bond cleavage, results in the mature form of the protein that gets secreted. Therefore, we chose to model both the pre-cleaved PROS1 (676 AA), and the truncated mature PROS1 (635 AA).
676 Precursor Protein Sequence
MRVLGGRCGALLACLLLVLPVSEANFLSKQQASQVLVRKRRANSLLEETKQGNLERECIEELCNKEEARE
VFENDPETDYFYPKYLVCLRSFQTGLFTAARQSTNAYPDLRSCVNAIPDQCSPLPCNEDGYMSCKDGKASFTCTCKPGWQGEKCEFDINECKDPSNIN
GGCSQICDNTPGSYHCSCKNGFVMLSNKKDCKDVDECSLKPSICGTAVCKNIPGDFECECPEGYRYNLKSKSCEDIDECSENMCAQLCVNYPGGYTCYCDGKKGFKLAQ
DQKSCEVVSVCLPLNLDTKYELLYLAEQFAGVVLYLKFRLPEISRFSAEFDFRTYDSEGVILYAESIDHSAWLLIALRGGKIEVQLKNEHTSKITTGGDVINNGLWNMVSVEELE
HSISIKIAKEAVMDINKPGPLFKPENGLLETKVYFAGFPRKVESELIKPINPRLDGCIRSWNLMKQGASGIKEIIQEKQNKHCLVTVEKGSYYPGSGIAQFHIDYNNVSSAEGW
HVNVTLNIRPSTGTGVMLALVSGNNTVPFAVSLVDSTSEKSQDILLSVENTVIYRIQALSLCSDQQSHLEFRVNRNNLELSTPLKIETISHEDLQRQLAVLDKAMKAKVAT
YLGGLPDVPFSATPVNAFYNGCMEVNINGVQLDLDEAISKHNDIRAHSCPSVWKKTKNS
635 Mature Protein Sequence
ANSLLEETKQGNLERECIEELCNKEEAREVFENDPETDYFYPKYLVCLRSFQTGLFTAARQSTNAYPDLR
SCVNAIPDQCSPLPCNEDGYMSCKDGKASFTCTCKPGWQGEKCEFDINECKDPSNINGGCSQICDNTPGSYHCSCKNGFVMLSNKKDCKDVDECSLKPSICGTAVCKNI
PGDFECECPEGYRYNLKSKSCEDIDECSENMCAQLCVNYPGGYTCYCDGKKGFKLAQDQKSCEVVSVCLPLNLDTKYELLYLAEQFAGVVLYLKFRLPEISRFSAEFDF
RTYDSEGVILYAESIDHSAWLLIALRGGKIEVQLKNEHTSKITTGGDVINNGLWNMVSVEELEHSISIKIAKEAVMDINKPGPLFKPENGLLETKVYFAGFPRKVESEL
IKPINPRLDGCIRSWNLMKQGASGIKEIIQEKQNKHCLVTVEKGSYYPGSGIAQFHIDYNNVSSAEGWHVNVTLNIRPSTGTGVMLALVSGNNTVPFAVSLVDSTSEKS
QDILLSVENTVIYRIQALSLCSDQQSHLEFRVNRNNLELSTPLKIETISHEDLQRQLAVLDKAMKAKVATYLGGLPDVPFSATPVNAFYNGCMEVNINGVQLDLDEAIS
KHNDIRAHSCPSVWKKTKNS
(1.)676 Precursor Protein Sequence model:
(2.)635 Precursor Protein Sequence model:
Ramachandran plots
The Ramachandran plot shows the statistical distribution of the combinations of the backbone dihedral angles ϕ and ψ. It gives information about the energetically allowed and disallowed regions in the protein. Having a lower number of residues in the disallowed regions and a high number of residues in the allowed energy regions indicates a good protein structure. We measured this parameter across all models obtained from the ab-initio method. The Ramachandran plots have been generated using MolProbity. It is most complete for crystal structure of proteins and acts as an active validation tool that produces coordinates, graphics and numerical evaluations. Higher weightage was given to the Ramachandran Plot values over the RMSD values.
(3.)Ramachandran Plot for the 676 Precursor Protein Sequence:
(4.)Ramachandran plot for the 635 Precursor Protein Sequence:
Conclusion
Through protein modeling, we were better able to understand the behavior of protein S, allowing us to understand how cells function and how the misfolded protein can cause disease. This data proved useful in allowing us to understand how the protein structure can change with different genetic mutations, and how this contributes to type I, type II, and type III protein S deficiency.
The protein modeling also gave us better insight into possible mutations that may arise when trying to express a recombinant version of the protein, and what the possible variations of our product might be from natural, functional protein S. Overall, generating possible structures provided us with a greater level of understanding of how protein S works, allowing us to create hypotheses about how to affect, control, and modify it. Even in the future, beyond iGEM, knowing the protein’s structure can also allow us to possibly design site-directed mutations with the intent of changing the protein function. We also plan on looking into the interaction between protein S and protein C, its binding partner, and using protein modeling to help predict their activity. This modeling provides an important first step that offers invaluable insight into our protein structure, function, and molecular dynamics.
Mathematical modeling
Many biological projects require models that can accurately represent and predict multicomponent, temporally evolving, dynamic systems. Differential equation models are often used for this purpose. This method models the interaction of molecules in the form of rate equations. The system is represented by a system of ordinary differential equations (ODEs), which quantify the interaction between different molecules (i.e. DNA, mRNA or protein), by using the law of mass action. These equations include terms relating to the binding of transcription factors and RNA polymerase to DNA, interactions between transcription factors, mRNA translation rate, and mRNA and protein degradation rates (Ay and Arnosti 2011), for example. In order to accomplish this, knowledge about the system components and structure is required.
We used MATLAB simulations to model our genetic circuits. We used a system of ODEs derived for both constitutive and regulatory genetic circuits(PPBP-Polyhedrin promoter interactions) for our SF9 expression system. We were able to input our system of ODE’s into MATLAB to predict our steady state concentrations prior to starting wet lab in order to determine the process that would yield more favorable results. Those plots can be found below:
SF9 constitutive gene circuit model:
(1.) Protein vs time:
(2.) mRNA vs time:
(3.) Effects of altering DNA concentration on protein production:
SF9 Regulatory gene circuit model:
The majority of recombinant protein expression is driven by the most powerful baculovirus promoter, polyhedrin, which is active in the late and very late stages of infection. Several studies have been done to study the mechanism of the polyhedrin promoter to better characterize its transcription activity. A host secreted transcription factor, polyhedrin promoter binding protein (PPBP) is known to bind a specific sequence on the polyhedrin promoter with extremely high affinity and specificity and plays a major role in the level of transcription through the polyhedrin promoter. It is established that the PPBP binds to the minor groove of DNA, interacting with the polyhedrin promoter sequence and forming a complex. Further studying the PPBP-DNA interactions and manipulating the concentration of PPBP in host SF9 cells can have a significant impact on the yield of recombinant proteins.
Here, we modeled the interaction between the PPBP and the polyhedrin promoter and its effect on the rate of production of mRNA and the resulting protein S. We were unable to find an established literature value for the concentration/number of molecules of the PPBP in SF9 cells, therefore, we estimated the concentration of PPBP based on some commonly found transcription factors in Drosophila Melanogaster. The plots of MATLAB simulations are listed below:
(1.) mRNA vs protein S over time:
(2.) mRNA vs time:
(3.) Protein vs time:
Information on parameter estimation, rate constants and analysis can be found on our model wiki page.
https://2022.igem.wiki/stony-brook/model
Summary: Modeling the sf9 regulatory gene circuit vs the sf9 constitutive gene circuit provides insight into the role of the Polyhedrin promoter binding protein and its effect on driving robust expression through the polyhedrin promoter. This is evident by the difference in the final steady-state protein values plotted by the regulatory vs the constitutive model. However, the exact dynamics of PPBP and polyhedrin promoter interactions are not well understood, it was difficult for us to find and approximate the literature values for initial steady state concentrations. Future models and experiments could seek to gain a better understanding of these values in order to make our simulation more accurate and improve our model.