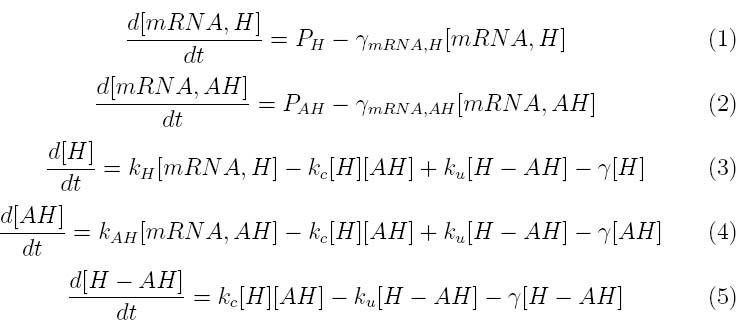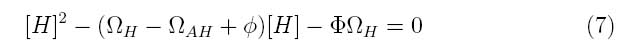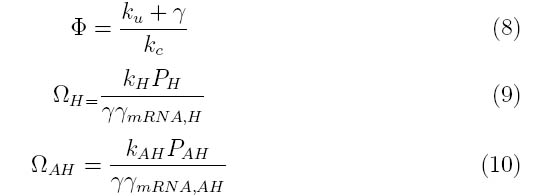Difference between revisions of "Part:BBa K112022"
(→Governing Equations) |
|||
| (4 intermediate revisions by the same user not shown) | |||
| Line 17: | Line 17: | ||
[[Image:K112019_data2.jpg|thumbnail|450px|center|Data from adding arabinose at midlog. Concentrations used at each data point from left to right are 0, 2.44E-07, 9.77E-07, 3.91E-06, 1.56E-05, 6.25E-05, 2.50E-04, and 1.00E-03 M]] | [[Image:K112019_data2.jpg|thumbnail|450px|center|Data from adding arabinose at midlog. Concentrations used at each data point from left to right are 0, 2.44E-07, 9.77E-07, 3.91E-06, 1.56E-05, 6.25E-05, 2.50E-04, and 1.00E-03 M]] | ||
| + | ==Modeling== | ||
| + | '''For more info, visit [http://2008.igem.org/Team:UC_Berkeley/LysisDevice UC Berkeley iGEM08 Wiki!!]'' | ||
| + | ===Motivation=== | ||
| + | |||
| + | Since several phage systems and many promoters are currently in use. Understanding the important parameters of the system allows one to choose appropriate promoters and phage proteins to optimize lysis behavior. Therefore we have created a steady-state kinetics model to describe the system. Our model is in agreement with our experimental results and published data regarding the T4 and λ phage systems. | ||
| + | |||
| + | ===Introduction=== | ||
| + | |||
| + | The holin-lysozyme phage lysis device consists of three proteins: | ||
| + | |||
| + | Holin - inner membrane-bound proteins that when complexed with other holin proteins, create holes in the membrane that allow lysozyme access to the periplasm. | ||
| + | |||
| + | Antiholin - inner membrane-bound (λ) or cytoplasmic (T4) protein that binds to and inactivates holin, producing an inactive holin-antiholin dimer. In the λ phage system, this dimer becomes active after holin forms enough pores in the plasma membrane to cause a loss of proton motive force between the periplasm and the cytosol. | ||
| + | |||
| + | Lysozyme - once holin forms pores in the inner membrane, lysozyme enters the periplasm and degrades peptidoglycan, resulting in cell lysis. | ||
| + | |||
| + | [[Image:Holinantiholin1.jpg]] | ||
| + | |||
| + | ===Governing Equations=== | ||
| + | |||
| + | Our lysis device consists of holin and lysozyme under an inducible promoter and antiholin under a constitutive promoter. The kinetics of our phage lysis device was modeled using first and second-order rate equations for the mRNAs and proteins of interest. The below equations describe our system | ||
| + | |||
| + | [[Image:Cdb1.jpg]] | ||
| + | |||
| + | where PH represent the holin mRNA promoter strength as a function of arabinose concentration and PAH represents the antiholin mRNA promoter strength. γmRNA,H and γmRNA,AH represent the degradation rates for holin and antiholin mRNAs respectively and γ represents the protein degradation rate. kH and kAH represent the rate constants for holin and antiholin protein translation and kc and ku represent the coupling and uncoupling rates for the holin-antiholin dimer. | ||
| + | |||
| + | ===Transfer Function and Dimensionless Parameters=== | ||
| + | |||
| + | At steady state, | ||
| + | |||
| + | [[Image:Cdb2.jpg]] | ||
| + | |||
| + | By making this assumption, the system of equations can be simplified into the following transfer function | ||
| + | |||
| + | [[Image:Cdb3.jpg]] | ||
| + | |||
| + | Where the system can be divided into three dimensionless parameters which describe the behavior of the holin-antiholin dimer and the holin and antiholin proteins. | ||
| + | |||
| + | [[Image:Cdb4.jpg]] | ||
| + | |||
| + | These dimensionless numbers can assist in the optimization of lysis device design because they describe the important parameters in the system. | ||
| + | |||
| + | For example, ΩH consists of the rate constants for the formation of holin mRNA and protein divided by their degradation rates. Strong promoters on holin will increase the value of ΩH, while high protein degradation rates will decrease ΩH. | ||
| + | |||
| + | ΩAH describes the rate constants and degradation rates for antiholin. | ||
| + | |||
| + | Φ describes the importance of the coupling and uncoupling rates of holin-antiholin dimer as well as the degradation rate of the dimer. | ||
| + | |||
| + | ===Graphs=== | ||
| + | |||
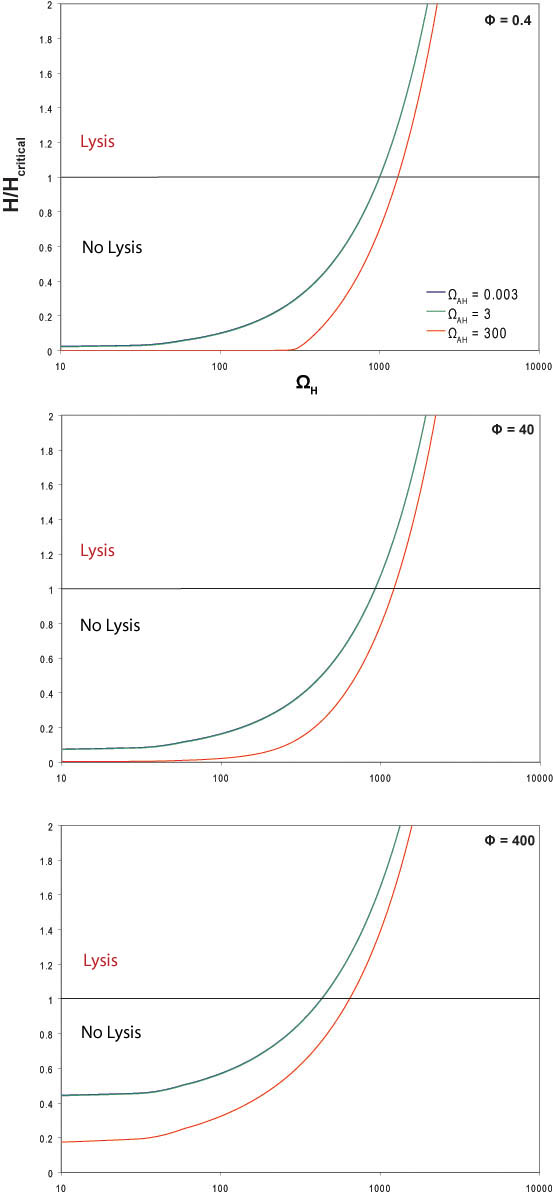
| + | Physiologically relevant values for ΩH, ΩAH and Φ were estimated based on rate constants for similar proteins. This system was input into MatLab to produce the following graph. Since there is a degree of uncertainty in these estimates, the graph spans several orders of magnitude above and below our estimated values. For the MatLab code used to produce these graphs, please click here: Matlab code. | ||
| + | |||
| + | [[Image:model6.jpg]] | ||
| + | |||
| + | The literature indicates that at the time of lysis, cells infected with λ phage have approximately 1000 holin proteins1. Therefore, the critical concentration of holin (Hc) was set at 1000 holin proteins per cell. | ||
| + | |||
| + | The horizontal line at y=1 represents the critical holin concentration needed induce lysis. If the system produces more than the critical concentration of holin (>1000 free holin proteins), lysis is expected to occur. | ||
| + | |||
| + | As one would expect by looking at the dimensionless value for omegaH, as the strength of the holin promoter increases, the amount of holin at steady state increases. | ||
| + | |||
| + | The graph also shows that the system is not very sensitive to small amounts of antiholin. Larger amounts of antiholin push the system equilibrium to the left and would require a stronger promoter on holin or a weaker binding interaction between holin and antiholin to reach critical concentration. This is supported by the observation that in a system with no antiholin, lysis can occur with holin under a weaker promoter. | ||
| + | |||
| + | Varying Φ, the dimensionless parameter that describes the coupling and uncoupling behavior of the holin-antiholin dimer, reveals that when the binding of holin-antiholin is stronger, a stronger promoter is required to reach the critical holin concentration. | ||
| + | |||
| + | ===Conclusions=== | ||
| + | |||
| + | There are several aspects of the model that agree with our experimental results and published data regarding phage lysis: | ||
| + | |||
| + | 1. Our experimental results show that at higher concentrations of arabinose, lysis occurs more readily. In our model, higher concentrations of arabinose increase the value of PH and thus increase the value of omegaH. Our model predicts that as omegaH increases, lysis is more likely to occur. | ||
| + | |||
| + | 2. Our experimental results show that the T4 lysis device results in lysis at a lower concentration of arabinose than our λ lysis device when the systems are under the same promoter. λ antiholin and holin should have similar degradation rates because both are membrane-bound and only differ by two amino acids2. In contrast, T4 antiholin is an unstable cytoplasmic protein with a rapid degradation rate (t1/2 = 2 min)3. Therefore γ should be larger in the T4 system, resulting in a larger value for Φ. This will shift the graph up and allow the system to reach the critical holin concentration more readily (see graph with Φ =400). | ||
| + | |||
| + | 3. Our model shows that systems with low values of omegaAH (weaker antiholin mRNA or protein promoter) will lyse more readily than systems with large values of omegaAH. This is in agreement with published results that show that in cells with little or no antiholin, lysis occurs more readily 2. | ||
| + | |||
| + | ===References=== | ||
| + | 1. Christos G. Savva, Jill S. Dewey, John Deaton, Rebecca L. White, Douglas K. Struck, Andreas Holzenburg and Ry Young. The holin of bacteriophage lambda forms rings with large diameter. Molecular Microbiology 69(4), 784–793. 2008. | ||
| + | |||
| + | 2. Martin Steiner and Udo Bläs. Charged amino-terminal amino acids affect the lethal capacity of Lambda lysis proteins S107 and S105. Molecular Microbiology. 8(3), 525 - 533. Oct. 2006. | ||
| + | |||
| + | 3. Tram Anh T. Tran, Douglas K. Struck, and Ry Young*. The T4 RI Antiholin Has an N-Terminal Signal Anchor Release Domain That Targets It for Degradation by DegP. Journal of Bacteriology. 7618–7625. Nov. 2007. | ||
---- | ---- | ||
Latest revision as of 16:29, 6 November 2008
Lambda phage lysis device - no promoter
This variation uses PconC5 to drive the production of antiholin.
Characterization
For more info, visit [http://2008.igem.org/Team:UC_Berkeley/LysisDevice UC Berkeley iGEM08 Wiki!!]
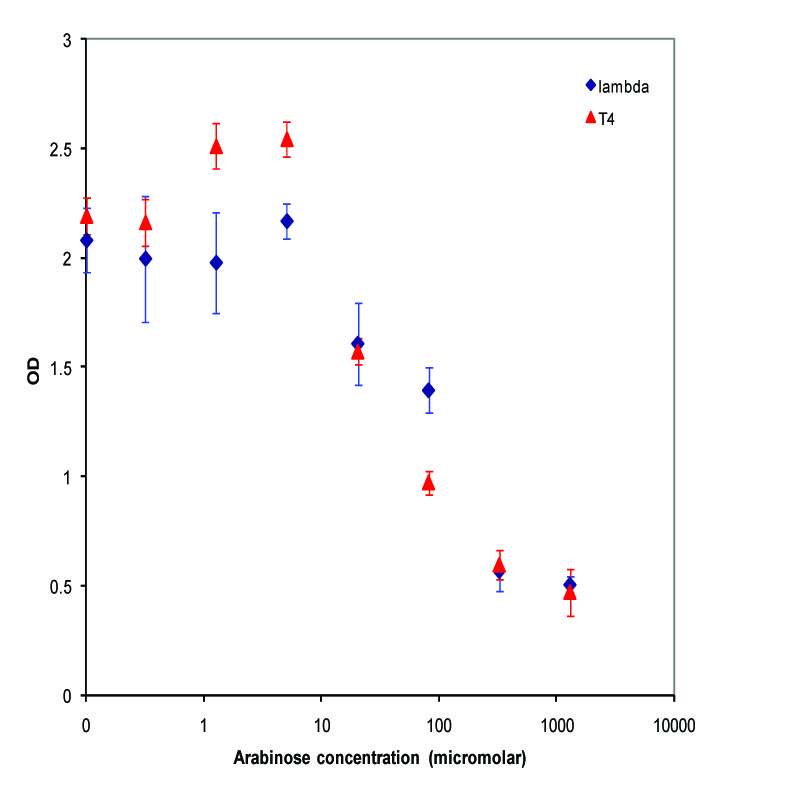
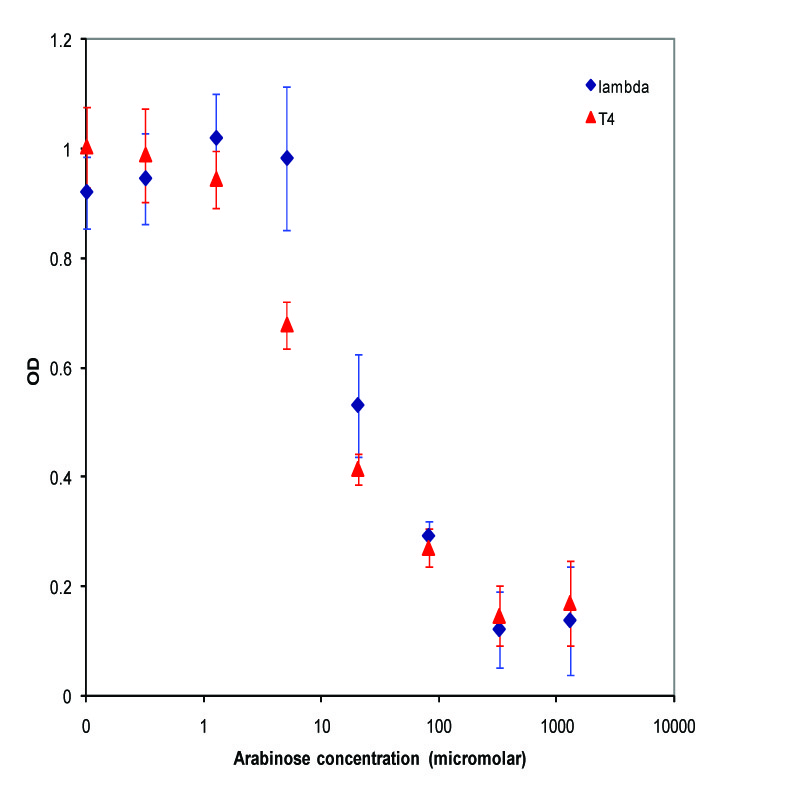
K112022 (Lambda Lysis Device) was assembled with a Pbad promoter and inserted in the vector K112950 and K112809 was inserted in pSB1A2. They were introduced into the MC1061 strain by transformation, and then picked 5 colonies for each device. We grew these cultures to saturation at 37 degrees Celsius in LB media, and then split into eight 1 mL aliquots. A range of concentrations of arabinose was added to these aliquots, with a starting concentration of 1.3E-3 M and the next 6 samples recieving a four-fold dilution of the previous sample, and an equal volume of water added to the last aliquot. The cultures were then incubated at 37 degrees again for 3.5 hours, and the absorbance at 600nm was measured with a Tecan Xfluor4 Safire2 in a Corning Inc. Costar 3603 plate. The data plotted on a log scale is shown below.
A parallel experiment was performed by taking the same saturated culture before induction with arabinose, diluting 100-fold, growing to mid-log(starting OD .22), before inducing with arabinose as above. The data plotted on a log scale is shown below.
Modeling
'For more info, visit [http://2008.igem.org/Team:UC_Berkeley/LysisDevice UC Berkeley iGEM08 Wiki!!]
Motivation
Since several phage systems and many promoters are currently in use. Understanding the important parameters of the system allows one to choose appropriate promoters and phage proteins to optimize lysis behavior. Therefore we have created a steady-state kinetics model to describe the system. Our model is in agreement with our experimental results and published data regarding the T4 and λ phage systems.
Introduction
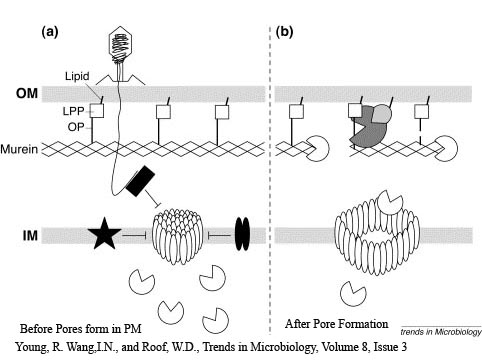
The holin-lysozyme phage lysis device consists of three proteins:
Holin - inner membrane-bound proteins that when complexed with other holin proteins, create holes in the membrane that allow lysozyme access to the periplasm.
Antiholin - inner membrane-bound (λ) or cytoplasmic (T4) protein that binds to and inactivates holin, producing an inactive holin-antiholin dimer. In the λ phage system, this dimer becomes active after holin forms enough pores in the plasma membrane to cause a loss of proton motive force between the periplasm and the cytosol.
Lysozyme - once holin forms pores in the inner membrane, lysozyme enters the periplasm and degrades peptidoglycan, resulting in cell lysis.
Governing Equations
Our lysis device consists of holin and lysozyme under an inducible promoter and antiholin under a constitutive promoter. The kinetics of our phage lysis device was modeled using first and second-order rate equations for the mRNAs and proteins of interest. The below equations describe our system
where PH represent the holin mRNA promoter strength as a function of arabinose concentration and PAH represents the antiholin mRNA promoter strength. γmRNA,H and γmRNA,AH represent the degradation rates for holin and antiholin mRNAs respectively and γ represents the protein degradation rate. kH and kAH represent the rate constants for holin and antiholin protein translation and kc and ku represent the coupling and uncoupling rates for the holin-antiholin dimer.
Transfer Function and Dimensionless Parameters
At steady state,
By making this assumption, the system of equations can be simplified into the following transfer function
Where the system can be divided into three dimensionless parameters which describe the behavior of the holin-antiholin dimer and the holin and antiholin proteins.
These dimensionless numbers can assist in the optimization of lysis device design because they describe the important parameters in the system.
For example, ΩH consists of the rate constants for the formation of holin mRNA and protein divided by their degradation rates. Strong promoters on holin will increase the value of ΩH, while high protein degradation rates will decrease ΩH.
ΩAH describes the rate constants and degradation rates for antiholin.
Φ describes the importance of the coupling and uncoupling rates of holin-antiholin dimer as well as the degradation rate of the dimer.
Graphs
Physiologically relevant values for ΩH, ΩAH and Φ were estimated based on rate constants for similar proteins. This system was input into MatLab to produce the following graph. Since there is a degree of uncertainty in these estimates, the graph spans several orders of magnitude above and below our estimated values. For the MatLab code used to produce these graphs, please click here: Matlab code.
The literature indicates that at the time of lysis, cells infected with λ phage have approximately 1000 holin proteins1. Therefore, the critical concentration of holin (Hc) was set at 1000 holin proteins per cell.
The horizontal line at y=1 represents the critical holin concentration needed induce lysis. If the system produces more than the critical concentration of holin (>1000 free holin proteins), lysis is expected to occur.
As one would expect by looking at the dimensionless value for omegaH, as the strength of the holin promoter increases, the amount of holin at steady state increases.
The graph also shows that the system is not very sensitive to small amounts of antiholin. Larger amounts of antiholin push the system equilibrium to the left and would require a stronger promoter on holin or a weaker binding interaction between holin and antiholin to reach critical concentration. This is supported by the observation that in a system with no antiholin, lysis can occur with holin under a weaker promoter.
Varying Φ, the dimensionless parameter that describes the coupling and uncoupling behavior of the holin-antiholin dimer, reveals that when the binding of holin-antiholin is stronger, a stronger promoter is required to reach the critical holin concentration.
Conclusions
There are several aspects of the model that agree with our experimental results and published data regarding phage lysis:
1. Our experimental results show that at higher concentrations of arabinose, lysis occurs more readily. In our model, higher concentrations of arabinose increase the value of PH and thus increase the value of omegaH. Our model predicts that as omegaH increases, lysis is more likely to occur.
2. Our experimental results show that the T4 lysis device results in lysis at a lower concentration of arabinose than our λ lysis device when the systems are under the same promoter. λ antiholin and holin should have similar degradation rates because both are membrane-bound and only differ by two amino acids2. In contrast, T4 antiholin is an unstable cytoplasmic protein with a rapid degradation rate (t1/2 = 2 min)3. Therefore γ should be larger in the T4 system, resulting in a larger value for Φ. This will shift the graph up and allow the system to reach the critical holin concentration more readily (see graph with Φ =400).
3. Our model shows that systems with low values of omegaAH (weaker antiholin mRNA or protein promoter) will lyse more readily than systems with large values of omegaAH. This is in agreement with published results that show that in cells with little or no antiholin, lysis occurs more readily 2.
References
1. Christos G. Savva, Jill S. Dewey, John Deaton, Rebecca L. White, Douglas K. Struck, Andreas Holzenburg and Ry Young. The holin of bacteriophage lambda forms rings with large diameter. Molecular Microbiology 69(4), 784–793. 2008.
2. Martin Steiner and Udo Bläs. Charged amino-terminal amino acids affect the lethal capacity of Lambda lysis proteins S107 and S105. Molecular Microbiology. 8(3), 525 - 533. Oct. 2006.
3. Tram Anh T. Tran, Douglas K. Struck, and Ry Young*. The T4 RI Antiholin Has an N-Terminal Signal Anchor Release Domain That Targets It for Degradation by DegP. Journal of Bacteriology. 7618–7625. Nov. 2007.
This part is in BBb Format. It is flanked by BamHI and BglII sites instead of XbaI and SpeI. More information about the BBb Format is available at:
[http://openwetware.org/wiki/Template:AndersonLab:BBb_Standard BBb Standard Description Page]
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 999
Illegal NheI site found at 1022 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 1385
- 1000COMPATIBLE WITH RFC[1000]