Difference between revisions of "Part:BBa K3610030"
(→References) |
(→Confocal Fluorescence Microcopy) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 38: | Line 38: | ||
Fluorescence Microscopy suggests that there is some expression of the BAK+ construct. It fails, however, to get localized at the cell periphery. Nevertheless, expression of the full length receptor in <i>S. cerevisiae</i> can already be seen as a considerable success. | Fluorescence Microscopy suggests that there is some expression of the BAK+ construct. It fails, however, to get localized at the cell periphery. Nevertheless, expression of the full length receptor in <i>S. cerevisiae</i> can already be seen as a considerable success. | ||
| + | ====Spectrometry==== | ||
| + | In addition to analyzing the cells with a microscope, we conducted a fluorescence assay with a plate reader. We conducted this experiment for multiple receptors at the same time. This way we were able to compare the expression levels of different versions of the BAK1 receptor. | ||
| + | For each receptor we tried to isolate three different biological samples, however, not all cells grew. | ||
| + | Ultimately, we only had two samples for the following <i>S. cerevisiae</i> cells: untransformed (Control), transformed with BAK1 ectodomain fused to YFP (eBAK) and the CORE ectodomain fused to YFP (eCORE). | ||
| + | For the BAK1 with and without the native signal peptide fused to YFP (BAK+ and BAK-) and the EFR ectodomain fused to YFP (eEFR), we had samples from three different colonies. | ||
| + | For each biological replicate, the optical density at absorbance of 600 nm (OD600) and the fluorescence levels were measured three times. | ||
| + | |||
| + | {| class="wikitable" width="95%" | ||
| + | |- | ||
| + | ! scope="col", colspan ="10"| measured OD600 values (OD) | ||
| + | |- | ||
| + | ! scope="col"| | ||
| + | ! scope="col", colspan="3"| Replicate 1 | ||
| + | ! scope="col", colspan="3"| Replicate 2 | ||
| + | ! scope="col", colspan="3"| Replicate 3 | ||
| + | |- | ||
| + | ! scope="row"| Blank | ||
| + | |0,08200000226||0,08200000226||0,08389999717|||||||||||| | ||
| + | |- | ||
| + | !Control | ||
| + | |0,3806000054||0,3747999966||0,4221999943||0,1316999942||0,131400004||0,1176000014|||||| | ||
| + | |- | ||
| + | !BAK+ | ||
| + | |0,4943000078||0,4638999999||0,4514000118||0,5781000257||0,5253999829||0,5799999833||0,2615999877||0,2171999961||0,2011999935 | ||
| + | |- | ||
| + | !BAK- | ||
| + | |1,417099953||1,365499973||1,368899941||0,6305999756||0,5633999705||0,6216999888||0,896600008||0,7882999778||0,8032000065 | ||
| + | |- | ||
| + | !eBAK | ||
| + | |1,009699941||0,8404999971||0,8934999704||0,2653000057||0,2368000001||0,2592999935|||||| | ||
| + | |- | ||
| + | !eCORE | ||
| + | |1,021499991||0,8616999984||0,9178000093||0,826300025||0,6888999939||0,7401999831|||||| | ||
| + | |- | ||
| + | !eEFR | ||
| + | |1,379699945||1,322700024||1,333500028||1,035899997||1,014000058||0,9526000023||0,4860999882||0,3797000051||0,3829999864 | ||
| + | |} | ||
| + | |||
| + | The following settings were applied for fluorescence measurements: | ||
| + | |||
| + | {| | ||
| + | |Mode: ||Fluorescence Top Reading | ||
| + | |- | ||
| + | |Excitation Wavelength: ||485 nm | ||
| + | |- | ||
| + | |Emission Wavelength: ||535 nm | ||
| + | |- | ||
| + | |Excitation Bandwidth: ||20 nm | ||
| + | |- | ||
| + | |Emission Bandwidth: ||25 nm | ||
| + | |- | ||
| + | |Temperature: ||22.3°C | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" width="95%" | ||
| + | |- | ||
| + | ! scope="col", colspan ="10"| Fluorescence Top Reading (FTR) | ||
| + | |- | ||
| + | ! scope="col"| | ||
| + | ! scope="col", colspan="3"| Replicate 1 | ||
| + | ! scope="col", colspan="3"| Replicate 2 | ||
| + | ! scope="col", colspan="3"| Replicate 3 | ||
| + | |- | ||
| + | ! scope="row"| Blank | ||
| + | |1297||1282||1322|||||||||||| | ||
| + | |- | ||
| + | !Control | ||
| + | |2684||2474||2634||1852||1792||1750|||||| | ||
| + | |- | ||
| + | !BAK+ | ||
| + | |3038||2813||2760||2836||2493||2788||2084||2072||2067 | ||
| + | |- | ||
| + | !BAK- | ||
| + | |35794||30319||31424||10792||9097||10517||22609||20227||21220 | ||
| + | |- | ||
| + | !eBAK | ||
| + | |26455||19828||21613||6614||5507||6229|||||| | ||
| + | |- | ||
| + | !eCORE | ||
| + | |10709||8382||9339||8957||7062||7735|||||| | ||
| + | |- | ||
| + | !eEFR | ||
| + | |43125||37782||39589||25641||24668||22517||12410||9054||9027 | ||
| + | |} | ||
| + | |||
| + | After measurement of the optical density and the fluorescence, the data were <i>blank corrected</i> (the average of the three blank measurements was subtracted from each measurement value). | ||
| + | |||
| + | The average of each of the three (or two) samples was calculated. From these values, the average was taken again. | ||
| + | |||
| + | After this step, we normalized the fluorescent output for OD600 (FTR/OD). | ||
| + | The results of these calculations are displayed in the table below. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | !Control | ||
| + | !BAK+ | ||
| + | !BAK- | ||
| + | !eBAK | ||
| + | !eCORE | ||
| + | !eEFR | ||
| + | |- | ||
| + | |4185,221063||9731,614266||26067,19254||28118,24739||3712,946478||23379,84399 | ||
| + | |} | ||
| + | |||
| + | If we set the values for the Control to 1 (Control = 1), then we get the fluorescence levels relative to the control, which is again diplayed in the table below. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | !Control | ||
| + | !eCORE | ||
| + | !eEFR | ||
| + | !BAK- | ||
| + | !BAK+ | ||
| + | !eBAK | ||
| + | |- | ||
| + | |1||0,8871565975||5,586286516||6,228390841||2,325233033||6,718461693 | ||
| + | |} | ||
| + | |||
| + | <br> | ||
| + | [[File:T--UZurich--Spectrometer1.png|500px|thumb|none|left|Figure 5: Fluorescence values standardized for OD600 of the different receptors (C=Control). Cells with BAK+ showed only weak fluorescence, while BAK-, eBAK and eEFR showed a strong increase in the fluorescence levels. CORE did not display any increase when compared with untreated <i>S. cerevisiae</i> cells (autofluorescence).]] | ||
| + | |||
| + | Fluorometric analysis of the sample showed increased fluorescence which suggests that the plasmid containing the part was expressed in <i>S. cerevisiae</i>. These results were also confirmed with fluorescent microscopy. | ||
| + | |||
| + | ====Flow Cytometry==== | ||
| + | It has been important to us to examine a sample with different approaches simultaneously, which is why we were eager to also measure fluorescence intensity by flow cytometry. In a first phase, 100,000 cells were measured from each biological replicate (488/530 FITC channel in a BD FACSCanto II flow cytometer). | ||
| + | In the next phase, the biological replicates for each construct were pooled together and 200,000 cells from each sample were measured. | ||
| + | |||
| + | [[File:T--UZurich--FACs.png|600px|thumb|none|Figure 6: Left: single biological replicates. right: pooled samples. With the exception of the construct with eCORE, cells transfected with our constructs showed considerably higher overall fluorescence intensities than the negative control.]] | ||
| + | |||
| + | Flow catometry provided further evidence for expression of the construct in <i>S. cerevisiae</i>. Cells transfected with plasmids containing BAK- showed significantly increased fluorescence intensities when examined as single biological replicates, as well as when the replicates were pooled together in one sample. | ||
| + | |||
| + | During our project, expression of the BAK1 receptor with the original signal peptide, fused to YFP was confirmed with three different approaches. We used a fluorometric plate reader, which suggested increased fluorescence intesity and we also got similar results when measuring fluorescence with flow cytometry. In addition to these results, fluorescence imaging also showed slightly increased fluorescence but no colocalization with the membrane stain FM4-64. | ||
| Line 49: | Line 180: | ||
<partinfo>BBa_K3610030 parameters</partinfo> | <partinfo>BBa_K3610030 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| − | |||
==References== | ==References== | ||
Latest revision as of 02:13, 28 October 2020
BAK1/YFP
This part can be used for expressing the plant pattern recognition receptor BRI1-associated receptro kinase (BAK1) from Arabidopsis thaliana in S. cerevisiae. This part contains the full length receptor BAK1, including the sequence for the signal peptide native to A. thaliana (BAK+). To make expression of BAK1 visible and to test observe the localization in the cell, the BAK1 coding region has been fused to a yellow fluorescent protein by a 15 amino-acid long linker.
Contents
Usage and Biology
BAK1 is a cell surface receptor protein with an intracellular kinase domain and an extracellular ligand binding domain. The receptor is necessary for many functions in the plant like brassinosteroid signalling and it is also a critical player in plant immunity, as BAK1 interacts with many important cell surface receptors which perceive pathogen-associated molecular patterns (PAMPs). One example of these PAMPs is the 22-amino-acid peptide flg22 from flagellin which is recognized by the leucine-rich repeat receptor kinases flagellin-sensitive 2 (FLS2). Upon recognizing the flg22 peptide, FLS2 interacts with BAK1. This interaction drives the immune response of the plant.
In our project, we used this part to test the expression of the whole length receptor in S. cerevisiae, as well as to observe the localization of the protein within the cell. It is important to note that the protein coding domain of this part has its original signal sequence from A. thaliana.
Characterization
Expression of BAK1/YFP in S. cerevisiae
In a first step we inserted the single fragments making up this part into a plasmid with a gentamycin-3-acetyltransferase gene and transformed E. coli (DH10alpha) with the plasmids for amplification. In the next step we assembled the fragments in a plasmid with a spectinomycin acetyltransferase and amplified the plasmids again in the same E. coli strain. For this step we applied the techniques of Golden Gate Cloning to get the fragments in the right order into the plasmid. The restriction enzyme we chose was BsaI. For expressing this part consisting of YFP and the receptor protein, we initially intended to use promoters of different strength to get more quantitative data. Finally, we got the construct in a plasmid with a truncated version of the ADH1 promoter from S. cerevisiae. For termination, this part has the terminator sequence of the enolase 2 protein from S. cerevisiae. The plasmid also contained the TRP1 gene, which encodes phosphoribosylanthranilate isomerase, an enzyme that catalyzes the third step in tryptophan biosynthesis. This enabled us to use the same plasmid for expression in S. cerevisiae. We prepared a medium containing YNB and free amino acids, without tryptophan. S. cerevisiae cells (AP4) were transfected with the plasmid and then plated on the selective medium.
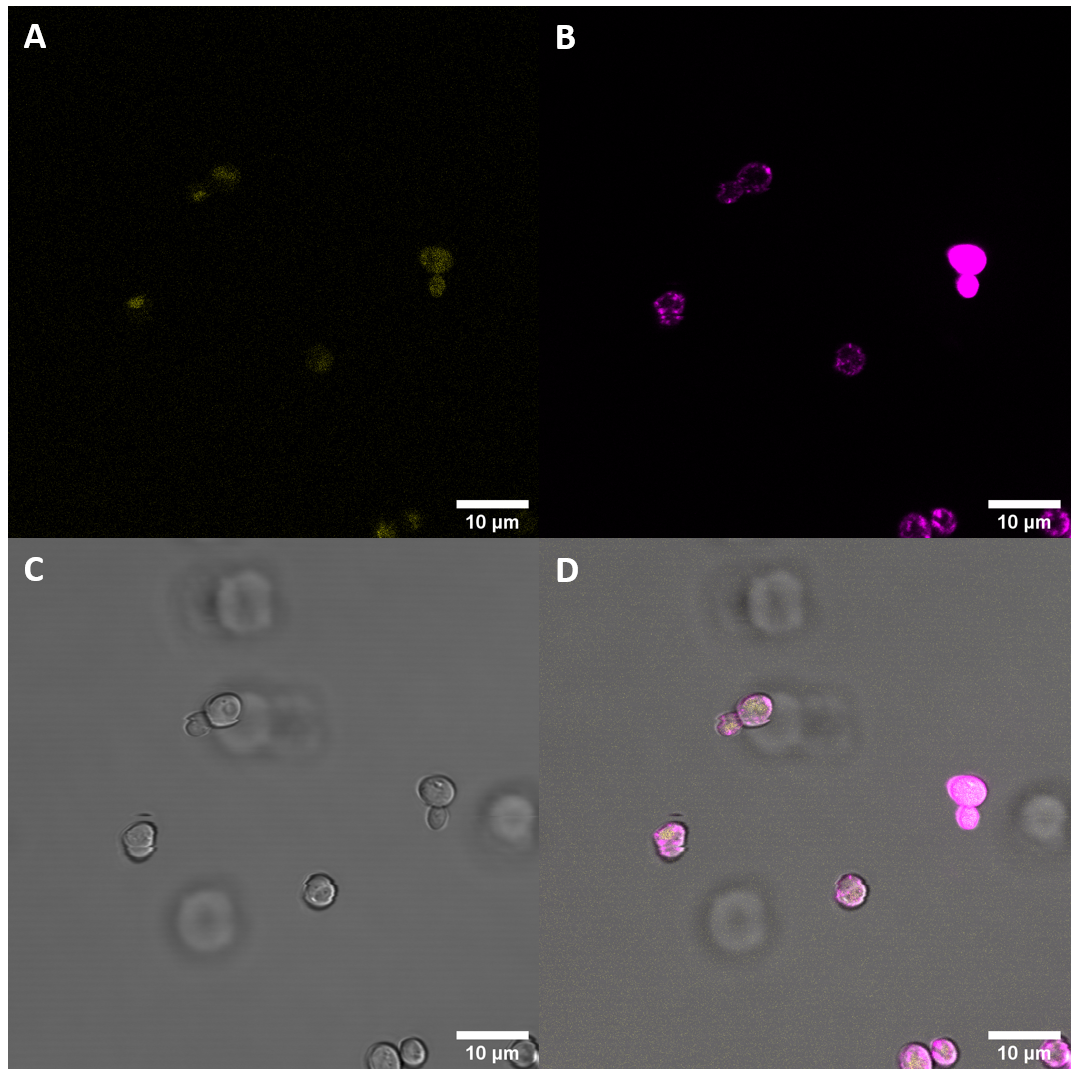
Confocal Fluorescence Microcopy
After successful transfection of S. cerevisiae cells with the plasmids we exmined the cell cultures with fluorescence microscopy.
Observed fluorescence with fluorescence microscopy was weak and did not appear to be localized at the plasma membrane (PM). As localization at the cell membrane was something we were particularily interested in, we repeated the confocal microscopy step with an additional membrane stain. The cell membrane was stained with fm4-64, which fluoresces strongly after binding to the cell membrane ((λEX = 515nm and λEM = 640nm). The binding of the dye is happening rapidly and it is also reversible. If the time spent between staining and imaging is too long, then the dye will be taken up by the organism and stored in the vacuole. Imaging with a confocal microscope for YFP and the fm4-64 stain shows the spatial overlap of the red fluorescence of the stain and the yellow fluorescence of the protein fused to the receptors.
Fluorescence Microscopy suggests that there is some expression of the BAK+ construct. It fails, however, to get localized at the cell periphery. Nevertheless, expression of the full length receptor in S. cerevisiae can already be seen as a considerable success.
Spectrometry
In addition to analyzing the cells with a microscope, we conducted a fluorescence assay with a plate reader. We conducted this experiment for multiple receptors at the same time. This way we were able to compare the expression levels of different versions of the BAK1 receptor. For each receptor we tried to isolate three different biological samples, however, not all cells grew. Ultimately, we only had two samples for the following S. cerevisiae cells: untransformed (Control), transformed with BAK1 ectodomain fused to YFP (eBAK) and the CORE ectodomain fused to YFP (eCORE). For the BAK1 with and without the native signal peptide fused to YFP (BAK+ and BAK-) and the EFR ectodomain fused to YFP (eEFR), we had samples from three different colonies. For each biological replicate, the optical density at absorbance of 600 nm (OD600) and the fluorescence levels were measured three times.
| measured OD600 values (OD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Replicate 1 | Replicate 2 | Replicate 3 | |||||||
| Blank | 0,08200000226 | 0,08200000226 | 0,08389999717 | ||||||
| Control | 0,3806000054 | 0,3747999966 | 0,4221999943 | 0,1316999942 | 0,131400004 | 0,1176000014 | |||
| BAK+ | 0,4943000078 | 0,4638999999 | 0,4514000118 | 0,5781000257 | 0,5253999829 | 0,5799999833 | 0,2615999877 | 0,2171999961 | 0,2011999935 |
| BAK- | 1,417099953 | 1,365499973 | 1,368899941 | 0,6305999756 | 0,5633999705 | 0,6216999888 | 0,896600008 | 0,7882999778 | 0,8032000065 |
| eBAK | 1,009699941 | 0,8404999971 | 0,8934999704 | 0,2653000057 | 0,2368000001 | 0,2592999935 | |||
| eCORE | 1,021499991 | 0,8616999984 | 0,9178000093 | 0,826300025 | 0,6888999939 | 0,7401999831 | |||
| eEFR | 1,379699945 | 1,322700024 | 1,333500028 | 1,035899997 | 1,014000058 | 0,9526000023 | 0,4860999882 | 0,3797000051 | 0,3829999864 |
The following settings were applied for fluorescence measurements:
| Mode: | Fluorescence Top Reading |
| Excitation Wavelength: | 485 nm |
| Emission Wavelength: | 535 nm |
| Excitation Bandwidth: | 20 nm |
| Emission Bandwidth: | 25 nm |
| Temperature: | 22.3°C |
| Fluorescence Top Reading (FTR) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Replicate 1 | Replicate 2 | Replicate 3 | |||||||
| Blank | 1297 | 1282 | 1322 | ||||||
| Control | 2684 | 2474 | 2634 | 1852 | 1792 | 1750 | |||
| BAK+ | 3038 | 2813 | 2760 | 2836 | 2493 | 2788 | 2084 | 2072 | 2067 |
| BAK- | 35794 | 30319 | 31424 | 10792 | 9097 | 10517 | 22609 | 20227 | 21220 |
| eBAK | 26455 | 19828 | 21613 | 6614 | 5507 | 6229 | |||
| eCORE | 10709 | 8382 | 9339 | 8957 | 7062 | 7735 | |||
| eEFR | 43125 | 37782 | 39589 | 25641 | 24668 | 22517 | 12410 | 9054 | 9027 |
After measurement of the optical density and the fluorescence, the data were blank corrected (the average of the three blank measurements was subtracted from each measurement value).
The average of each of the three (or two) samples was calculated. From these values, the average was taken again.
After this step, we normalized the fluorescent output for OD600 (FTR/OD). The results of these calculations are displayed in the table below.
| Control | BAK+ | BAK- | eBAK | eCORE | eEFR |
|---|---|---|---|---|---|
| 4185,221063 | 9731,614266 | 26067,19254 | 28118,24739 | 3712,946478 | 23379,84399 |
If we set the values for the Control to 1 (Control = 1), then we get the fluorescence levels relative to the control, which is again diplayed in the table below.
| Control | eCORE | eEFR | BAK- | BAK+ | eBAK |
|---|---|---|---|---|---|
| 1 | 0,8871565975 | 5,586286516 | 6,228390841 | 2,325233033 | 6,718461693 |

Fluorometric analysis of the sample showed increased fluorescence which suggests that the plasmid containing the part was expressed in S. cerevisiae. These results were also confirmed with fluorescent microscopy.
Flow Cytometry
It has been important to us to examine a sample with different approaches simultaneously, which is why we were eager to also measure fluorescence intensity by flow cytometry. In a first phase, 100,000 cells were measured from each biological replicate (488/530 FITC channel in a BD FACSCanto II flow cytometer). In the next phase, the biological replicates for each construct were pooled together and 200,000 cells from each sample were measured.
Flow catometry provided further evidence for expression of the construct in S. cerevisiae. Cells transfected with plasmids containing BAK- showed significantly increased fluorescence intensities when examined as single biological replicates, as well as when the replicates were pooled together in one sample.
During our project, expression of the BAK1 receptor with the original signal peptide, fused to YFP was confirmed with three different approaches. We used a fluorometric plate reader, which suggested increased fluorescence intesity and we also got similar results when measuring fluorescence with flow cytometry. In addition to these results, fluorescence imaging also showed slightly increased fluorescence but no colocalization with the membrane stain FM4-64.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal SpeI site found at 1560
Illegal PstI site found at 650
Illegal PstI site found at 695
Illegal PstI site found at 985 - 12INCOMPATIBLE WITH RFC[12]Illegal SpeI site found at 1560
Illegal PstI site found at 650
Illegal PstI site found at 695
Illegal PstI site found at 985 - 21COMPATIBLE WITH RFC[21]
- 23INCOMPATIBLE WITH RFC[23]Illegal SpeI site found at 1560
Illegal PstI site found at 650
Illegal PstI site found at 695
Illegal PstI site found at 985 - 25INCOMPATIBLE WITH RFC[25]Illegal SpeI site found at 1560
Illegal PstI site found at 650
Illegal PstI site found at 695
Illegal PstI site found at 985 - 1000COMPATIBLE WITH RFC[1000]
References
Chinchilla, Delphine; Zipfel, Cyril; Robatzek, Silke; Kemmerling, Birgit; Nürnberger, Thorsten; Jones, Jonathan D. G. et al. (2007): A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. In: Nature 448 (7152), S. 497–500. DOI: 10.1038/nature05999.
Macho, Alberto P.; Zipfel, Cyril (2014): Plant PRRs and the Activation of Innate Immune Signaling. In: Molecular Cell 54 (2), S. 263–272. DOI: 10.1016/j.molcel.2014.03.028.
Yan, Liming; Ma, Yuanyuan; Liu, Dan; Wei, Xiaochao; Sun, Yuna; Chen, Xiaoyue et al. (2012): Structural basis for the impact of phosphorylation on the activation of plant receptor-like kinase BAK1. In: Cell Res 22 (8), S. 1304–1308. DOI: 10.1038/cr.2012.74.
Rigal, A., Doyle, S. M., & Robert, S. (2015). Live cell imaging of FM4-64, a tool for tracing the endocytic pathways in Arabidopsis root cells. Methods in molecular biology (Clifton, N.J.), 1242, 93–103