Difference between revisions of "Chassis/B.subtilis Strains"
m (→168) |
(→Commonly used strains: (edited to add 2 strains: "KO7" ; "PDC204 and derivatives")) |
||
| (10 intermediate revisions by 4 users not shown) | |||
| Line 12: | Line 12: | ||
==Introduction== | ==Introduction== | ||
| + | |||
| + | [[Image:Bsub-1.gif|right|frame| ''B. subtilis'' swimming in LB medium]] | ||
| + | |||
| + | |||
| + | |||
| + | ''Bacillus subtilis'' is a commonly used laboratory bacterium. It is often considered the gram-positive ''E.coli'' because of the amount of use it has received and the amount of knowledge there is about the organism. | ||
| + | |||
| + | |||
| + | |||
| + | Its genome has been sequenced (as has the genome of at least one strain - 168) and it has been used at iGEM since 2007. It is gradually coming in to favour - particularly in the UK - as a potential host for many synthetic systems and devices, often due to the characteristics that make it different to ''E.coli''. | ||
| + | |||
| + | |||
| + | |||
| + | The lack of parts for ''B. subtilis'' on the registry have reduced the common use of ''B. subtilis'' as a chassis, but with the increased use of ''B. subtilis'', particularly by iGEM teams, it may be soon become a third major host for the registry. | ||
| Line 49: | Line 63: | ||
| style="background:#DAFBAA"| '''Sporulation: Transport''' - Under stress conditions, ''B. subtilis'' will form spores. These spores are highly resistant versions of single cells, able to withstand extreme temperatures and pH. These spores are capable of growing into new cells once favourable growing conditions are restored. Due to the resistance of spores, there is a great potential for manipulating them for transporting ''B. subtilis'' devices and constructs. | | style="background:#DAFBAA"| '''Sporulation: Transport''' - Under stress conditions, ''B. subtilis'' will form spores. These spores are highly resistant versions of single cells, able to withstand extreme temperatures and pH. These spores are capable of growing into new cells once favourable growing conditions are restored. Due to the resistance of spores, there is a great potential for manipulating them for transporting ''B. subtilis'' devices and constructs. | ||
|style="background:#fedddd"|'''Sporulation''' - Under stress conditions, ''B. subtilis'' will form spores. These spores are highly resistant versions of single cells. While ''B. subtilis'' itself isn't pathogenic, its spores can be damaging if inhaled. They are also highly resistant to many conditions that are used to kill cells. | |style="background:#fedddd"|'''Sporulation''' - Under stress conditions, ''B. subtilis'' will form spores. These spores are highly resistant versions of single cells. While ''B. subtilis'' itself isn't pathogenic, its spores can be damaging if inhaled. They are also highly resistant to many conditions that are used to kill cells. | ||
| − | |||
| − | |||
|- | |- | ||
|style="background:#DAFBAA"| '''Non-pathogenicity''' - ''B. subtilis'' is a non-pathogenic organism that is commonly found in soil. As a result, ''B. subtilis'' has a biological harzardous level of 1 and offers a useful non-pathogenic chassis for synthetic biologists. | |style="background:#DAFBAA"| '''Non-pathogenicity''' - ''B. subtilis'' is a non-pathogenic organism that is commonly found in soil. As a result, ''B. subtilis'' has a biological harzardous level of 1 and offers a useful non-pathogenic chassis for synthetic biologists. | ||
| + | |style="background:#fedddd"|'''Limited Cell Wall Permeability''' - ''B. subtilis'' has a thick peptidoglycan layer surrounding its single membrane. This may pose a problem to projects that require an interaction between large extracellular ligands and membrane-bound proteins. You can find more information and potential solutions to this issue at https://2020.igem.org/Team:UofUppsala/Contribution. | ||
| + | |- | ||
| + | |style="background:#DAFBAA"| '''High Motility''' - ''B.subtilis'' is often referred to as a highly motile organism in comparison to other bacterium. | ||
|} | |} | ||
<br> | <br> | ||
| Line 60: | Line 75: | ||
=====168===== | =====168===== | ||
| − | + | ''Bacillus subtilis'' strain 168 is one of the most commonly used in the laboratory and its specific genome has been sequenced. | |
168 tends to be the traditional gram-positive lab worhhorse. | 168 tends to be the traditional gram-positive lab worhhorse. | ||
| − | It was used by the Imperial 2008 iGEM team in | + | It was used by the Imperial 2008 iGEM team in the project Biofabricator ''subtilis'' and proved to be a useful and reliable chassis, being easy to transform, highly motile and generally easy to work with. It was also used by the Cambridge, Edinburgh and Newcastle teams in the same year for a range of applications. |
| + | |||
| + | This chassis currently provides the basis for the ''B. subtilis'' chassis characterisation data | ||
=====Py79===== | =====Py79===== | ||
| + | |||
| + | Like 168, Py79 is also a lab strain of ''Bacillus subtilis''. | ||
| + | |||
| + | It is commonly used in research and also is a general workhorse strain. | ||
| + | |||
| + | =====KO7===== | ||
| + | |||
| + | In recent years, ''B. subtilis'' rose in popularity within the industrial setting as a protein secretion chassis. This bacterial species is known to secrete a wide variety of proteins, making it a more desirable model organism for this purpose when compared to its gram-negative counterpart – ''E. coli''. This characteristic is also desirable in biosensors or other catalytic systems where one or more steps involves the secretion of a given protein. | ||
| + | |||
| + | However, ''B. subtilis'' also secretes a considerable number of proteases to the extracellular medium. These enzymes will end up reducing both the half-life and the total amount of secreted protein – an undesirable outcome of any biotechnological application. In an industrial process, this issue leads to significant economic losses; in the case of a biosensor, it reduces the sensitivity of the system. | ||
| + | |||
| + | Dr. Daniel R. Zeigler – the director of the Bacillus Genetic Stock Center – realized that a biotechnology project seeking a higher efficiency would naturally gravitate towards a ''B. subtilis'' strain where extracellular proteolytic activity is less prominent. Thus, he developed the ''B. subtilis'' KO7 strain, in which 7 genes for secreted proteases have been knocked out. You can learn more about this strain and other Bacillus-related products at http://www.bgsc.org/. | ||
| + | |||
| + | =====PDC204 and derivatives===== | ||
| + | |||
| + | These strains can easily be converted from the wildtype phenotype to the L-form state, which does not possess a cell wall. Thus, they are appropriate for usage in biotechnological applications where an interaction between a large extracellular ligand and a membrane-bound protein is required - such as in a biosensor. The operon MurE (involved in the creation of the cell wall) is modified to be under the control of a xylose-inducible promoter. This design implies that these bacteria will be devoid of a cell wall as long as they are grown in an osmotically stabilized medium without xylose.(1, 2) | ||
| + | |||
| + | 1. Wolf, D., Domínguez-Cuevas, P., Daniel, R. A., and Mascher, T. (2012) Cell Envelope Stress Response in Cell Wall-Deficient L-Forms of Bacillus subtilis. ''Antimicrob. Agents Chemother.'' '''56''', 5907–5915. | ||
| + | |||
| + | 2. Domínguez-Cuevas, P., Mercier, R., Leaver, M., Kawai, Y., and Errington, J. (2012) The rod to L-form transition of Bacillus subtilis is limited by a requirement for the protoplast to escape from the cell wall sacculus: L-form escape. ''Molecular Microbiology.'' '''83''', 52–66. | ||
Latest revision as of 16:14, 24 October 2020
Bacillus subtilis
| B.subtilis strains | B. subtilis chassis characterisation | B. subtilis specific parts | Key Parts and Devices |
Introduction
Bacillus subtilis is a commonly used laboratory bacterium. It is often considered the gram-positive E.coli because of the amount of use it has received and the amount of knowledge there is about the organism.
Its genome has been sequenced (as has the genome of at least one strain - 168) and it has been used at iGEM since 2007. It is gradually coming in to favour - particularly in the UK - as a potential host for many synthetic systems and devices, often due to the characteristics that make it different to E.coli.
The lack of parts for B. subtilis on the registry have reduced the common use of B. subtilis as a chassis, but with the increased use of B. subtilis, particularly by iGEM teams, it may be soon become a third major host for the registry.
Benefits vs Challenges
|
|
||
|---|---|---|
|
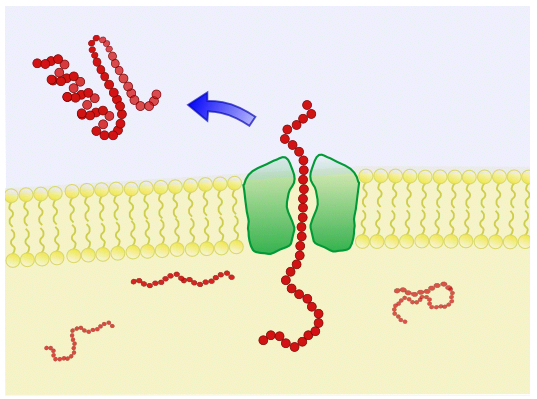
Single Membrane - B. subtilis is a gram-positive bacterium and contains only a single membrane. This makes B. subtilis an ideal chassis for secretion of organic molecules. | Peptidase activity - B. subtilis express both intracellular and extracellular exopeptidases and so there is a risk that expressed proteins may be cleaved up inside the cytoplasm or in the solution. |

|
Availability of Potential Parts - B. subtilis is a highly studied organism, with a fully sequenced genome. As a result, B. subtilis provides many useful potential parts. | B. subtilis parts - Although many labs and iGEM teams have looked at B. subtilis as a potential chassis, there are relatively few working B. subtilis parts available in the Registry. This however is rapidly changing and soon there will be the basic parts required to make B. subtilis a highly viable chassis. |
|
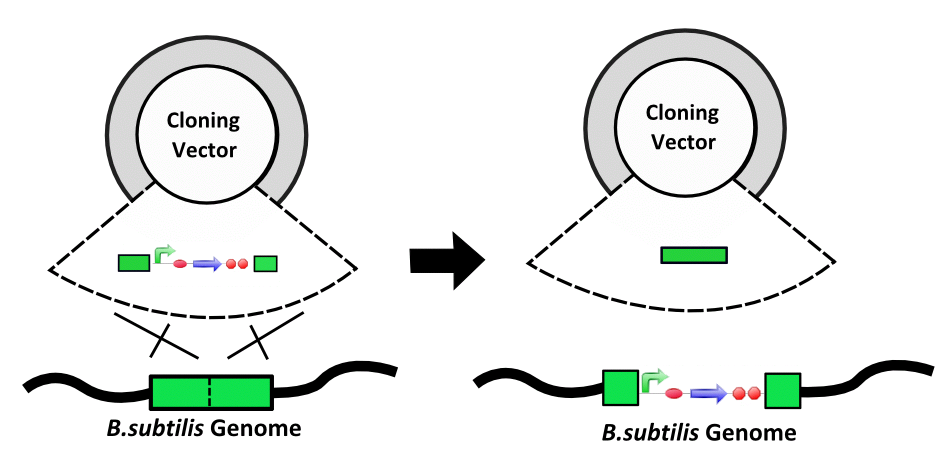
Natural Competency and Integration - B. subtilis has been noted for its ease and efficieny of transformation. In addition, integration of exogenous DNA into the chromosome has been well studied and provides an alternative to using traditional plasmids. | Vector Degradation - B. subtilis does not use all the same vectors as E. coli. One reason for this is that B. subtilis often recognises vectors grown in E. coli as foreign and digests them. Vectors and shuttle vectors are thus not reliable carriers of genetic information. |
|
Sporulation: Transport - Under stress conditions, B. subtilis will form spores. These spores are highly resistant versions of single cells, able to withstand extreme temperatures and pH. These spores are capable of growing into new cells once favourable growing conditions are restored. Due to the resistance of spores, there is a great potential for manipulating them for transporting B. subtilis devices and constructs. | Sporulation - Under stress conditions, B. subtilis will form spores. These spores are highly resistant versions of single cells. While B. subtilis itself isn't pathogenic, its spores can be damaging if inhaled. They are also highly resistant to many conditions that are used to kill cells. |
| Non-pathogenicity - B. subtilis is a non-pathogenic organism that is commonly found in soil. As a result, B. subtilis has a biological harzardous level of 1 and offers a useful non-pathogenic chassis for synthetic biologists. | Limited Cell Wall Permeability - B. subtilis has a thick peptidoglycan layer surrounding its single membrane. This may pose a problem to projects that require an interaction between large extracellular ligands and membrane-bound proteins. You can find more information and potential solutions to this issue at https://2020.igem.org/Team:UofUppsala/Contribution. | |
| High Motility - B.subtilis is often referred to as a highly motile organism in comparison to other bacterium. | ||
Commonly used strains
168
Bacillus subtilis strain 168 is one of the most commonly used in the laboratory and its specific genome has been sequenced.
168 tends to be the traditional gram-positive lab worhhorse.
It was used by the Imperial 2008 iGEM team in the project Biofabricator subtilis and proved to be a useful and reliable chassis, being easy to transform, highly motile and generally easy to work with. It was also used by the Cambridge, Edinburgh and Newcastle teams in the same year for a range of applications.
This chassis currently provides the basis for the B. subtilis chassis characterisation data
Py79
Like 168, Py79 is also a lab strain of Bacillus subtilis.
It is commonly used in research and also is a general workhorse strain.
KO7
In recent years, B. subtilis rose in popularity within the industrial setting as a protein secretion chassis. This bacterial species is known to secrete a wide variety of proteins, making it a more desirable model organism for this purpose when compared to its gram-negative counterpart – E. coli. This characteristic is also desirable in biosensors or other catalytic systems where one or more steps involves the secretion of a given protein.
However, B. subtilis also secretes a considerable number of proteases to the extracellular medium. These enzymes will end up reducing both the half-life and the total amount of secreted protein – an undesirable outcome of any biotechnological application. In an industrial process, this issue leads to significant economic losses; in the case of a biosensor, it reduces the sensitivity of the system.
Dr. Daniel R. Zeigler – the director of the Bacillus Genetic Stock Center – realized that a biotechnology project seeking a higher efficiency would naturally gravitate towards a B. subtilis strain where extracellular proteolytic activity is less prominent. Thus, he developed the B. subtilis KO7 strain, in which 7 genes for secreted proteases have been knocked out. You can learn more about this strain and other Bacillus-related products at http://www.bgsc.org/.
PDC204 and derivatives
These strains can easily be converted from the wildtype phenotype to the L-form state, which does not possess a cell wall. Thus, they are appropriate for usage in biotechnological applications where an interaction between a large extracellular ligand and a membrane-bound protein is required - such as in a biosensor. The operon MurE (involved in the creation of the cell wall) is modified to be under the control of a xylose-inducible promoter. This design implies that these bacteria will be devoid of a cell wall as long as they are grown in an osmotically stabilized medium without xylose.(1, 2)
1. Wolf, D., Domínguez-Cuevas, P., Daniel, R. A., and Mascher, T. (2012) Cell Envelope Stress Response in Cell Wall-Deficient L-Forms of Bacillus subtilis. Antimicrob. Agents Chemother. 56, 5907–5915.
2. Domínguez-Cuevas, P., Mercier, R., Leaver, M., Kawai, Y., and Errington, J. (2012) The rod to L-form transition of Bacillus subtilis is limited by a requirement for the protoplast to escape from the cell wall sacculus: L-form escape. Molecular Microbiology. 83, 52–66.

