Difference between revisions of "Part:BBa K3552000"
(added breaks in references) |
|||
| (20 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K3552000 short</partinfo> | <partinfo>BBa_K3552000 short</partinfo> | ||
| − | GsPilA is a type 4 pilus | + | Geobacter sulfurreducens pilA(GsPilA) is a type 4 pilus usually found on Geobacter sulfurreducens which can conduct electricity. This part is in the part collection where we provide different conductive pilus based on the same pilin generator. By these different type 4 pilus, we can compare the qualities of stability and yield. |
The part collection includes: | The part collection includes: | ||
| Line 35: | Line 35: | ||
<partinfo>BBa_K3552012</partinfo>. | <partinfo>BBa_K3552012</partinfo>. | ||
| − | Our part collection can instruct other teams to | + | Our part collection can instruct other teams to design new rechargeable pilus and substitute them to have more combinations that may achieve better performance in the future when manufacturing the batteries. |
<span class='h3bb'>'''Sequence and Features'''</span> | <span class='h3bb'>'''Sequence and Features'''</span> | ||
| Line 44: | Line 44: | ||
==Usage and Biology== | ==Usage and Biology== | ||
| − | GsPilA | + | GsPilA is a fimbrial protein that normally functions in Geobacter sulfurreducens which can conduct electricity. It is a type 4 pili that is long and thin, displaying on the cell surface membrane. It can promote adherence, motility, and transport functions in the bacteria. It is mainly built as helical polymers of a single subunit called the major pilin. These pilins are initially in the plasma membrane and the N-terminal is positively charged. The mature pilins can be extracted through the membrane by the pilin generator. |
| + | |||
| + | |||
| + | ==Structure and function== | ||
| + | The pili of <i>Geobacter sulfurreducens</i> are filaments primarily made up of the <i>PilA</i> protein monomer. Due to the short length of the monomer, aromatic residues (tryptophan, phenylalanine, and tyrosine) can be tightly packed along the pili’s length, providing it with conductive properties [1]. This characteristic can be explained by the phenomenon of pi stacking, in which the aromatic side chains align, creating a path for electron transfer to occur [2]. The relationship between the <i>PilA's</i> conductive properties and its protein sequence has further been proven by genetic engineering. For instance, conductivity of pili in which the five aromatic amino acids of the monomers are substituted with alanine is shown to drop to 38 ± 1 μS cm−1, three orders of magnitude lower than the native pili, underscoring the significance of the pi interactions in electron transfer [3]. Furthermore, pH also impacts the conductivity of pili; ranging from 51 mS/cm3 at pH 7 to 37 mS/cm3 at pH 10.5, with the highest conductivity value, 188 mS/cm3, observed at pH 2 [3]. Consistent with this, purified pili films demonstrate non-redox electronic conduction in a buffered aqueous environment; pili can conduct electrons without going through redox processes, which is in line with a metallic charge carrier transport model. This comes in sharp contrast to other organic reactions (e.g. DNA synthesis, photosynthesis), which follow a donor–bridge–acceptor model, where electric potential is passed on by the transfer of electrons from one molecule to the next until it reaches the final acceptor.This also explains how the conductivity of the pili is comparable to synthetic conductive materials, such as carbon nanotubes or silicon nanowires [4] [5]. | ||
| + | |||
| + | ==Novel research and potential applications== | ||
| + | Over the last decade, research on pili has revealed a number of potential applications for <i>G. sulfurreducens</i>. For instance, microbial fuel cells can be constructed by connecting a<i>G. sulfurreducens biofilm</i> with the organic waste (e.g. acetate), generating electricity through pili-mediated reduction, [6]. Similarly, the capacity of natural Geobacter to reduce and detoxify radioactive elements and heavy metals in contaminated environments through pili-mediated reductions (e.g soluble Uranium VI to Uranium IV, a form that precipitates and is distributed at a lower rate) can also be enhanced, creating more efficient systems for clearing radiologically and chemically contaminated areas [7] [8]. Electrical biosensors, which could have medical applications in the future for the detection of relevant metabolic compounds, can be created by modifying the C-terminal end of the pilin monomers. Hybrid pili, which consist of wild type, as well as his-tagged and HA-tagged monomers have been shown to conduct electricity better than the wild type pili, as well as change their conductivity potential when binding to the target molecule nickel [9] [10]. Finally, <i>G. sulfurreducens</i> can serve as a model organism for microbially-induced corrosion in metal structures, such as storage tanks and pipes; understanding the electrochemical mechanism of this process can pave the way for technologies that lower maintenance requirements and increase the longevity of these buildings [11]. | ||
| + | |||
| + | ==References (for ''Structure and function'' and ''Novel research and potential applications'')== | ||
| + | [1] Holmes DE, Dang Y, Walker DJF, Lovley DR. The electrically conductive pili of Geobacter species are a recently evolved feature for extracellular electron transfer. Microbial Genomics. 2016 Aug 25;2(8). | ||
| + | <br>[2] Vargas M, Malvankar NS, Tremblay PL, Leang C, Smith JA, Patel P, et al. Aromatic Amino Acids Required for Pili Conductivity and Long-Range Extracellular Electron Transport in <i>Geobacter sulfurreducens</i>. Giovannoni SJ, editor. mBio. 2013 May;4(2). | ||
| + | <br>[3] Adhikari RY, Malvankar NS, Tuominen MT, Lovley DR. Conductivity of individual Geobacter pili. RSC Advances. 2016 Jan 19;6(10):8354–7. | ||
| + | <br>[4] Ing NL, Nusca TD, Hochbaum AI. <i>Geobacter sulfurreducens</i> pili support ohmic electronic conduction in aqueous solution. Physical Chemistry Chemical Physics 2017 Jan 1;19(32):21791–9. | ||
| + | <br>[5] Ueki T, Walker DJF, Woodard TL, Nevin KP, Nonnenmann SS, Lovley DR. An <i>Escherichia coli</i> Chassis for Production of Electrically Conductive Protein Nanowires. ACS Synthetic Biology. 2020 Mar 3;9(3):647–54. | ||
| + | <br>[6] Nevin KP, Zhang P, Franks AE, Woodard TL, Lovley DR. Anaerobes unleashed: Aerobic fuel cells of <i>Geobacter sulfurreducens</i>. J Power Sources. 2011;196(18):7514–8. | ||
| + | <br>[7] Dang Y, Walker DJF, Vautour KE, Dixon S, Holmes DE. Arsenic Detoxification by Geobacter Species. Liu SJ, editor. Applied and Environmental Microbiology. 2017 Feb 15;83(4). | ||
| + | <br>[8] Cologgi DL, Speers AM, Bullard BA, Kelly SD, Reguera G. Enhanced Uranium Immobilization and Reduction by <i>Geobacter sulfurreducens</i> Biofilms. Parales RE, editor. Applied and Environmental Microbiology. 2014 Nov;80(21):6638–46 | ||
| + | <br>[9] Ueki T, Walker DJF, Tremblay PL, Nevin KP, Ward JE, Woodard TL, et al. Decorating the Outer Surface of Microbially Produced Protein Nanowires with Peptides. ACS Synthetic Biology. 2019 Jul 12;8(8):1809–17. | ||
| + | <br>[10] Eric Szmuc, David J.F. Walker, Dmitry Kireev, Deji Akinwande, Derek R. Lovley, Benjamin Keitz, Andrew Ellington. Engineering Geobacter pili to produce metal:organic filaments, Biosensors and Bioelectronics, Volume 222, 2023. | ||
| + | <br>[11] Liu X, Walker DJF, Nonnenmann SS, Sun D, Lovley DR. Direct Observation of Electrically Conductive Pili Emanating from <i>Geobacter sulfurreducens</i>. Papoutsakis ET, editor. mBio. 2021 Aug 31;12(4). | ||
| + | |||
==Characterization== | ==Characterization== | ||
| − | |||
| − | + | ===Construction=== | |
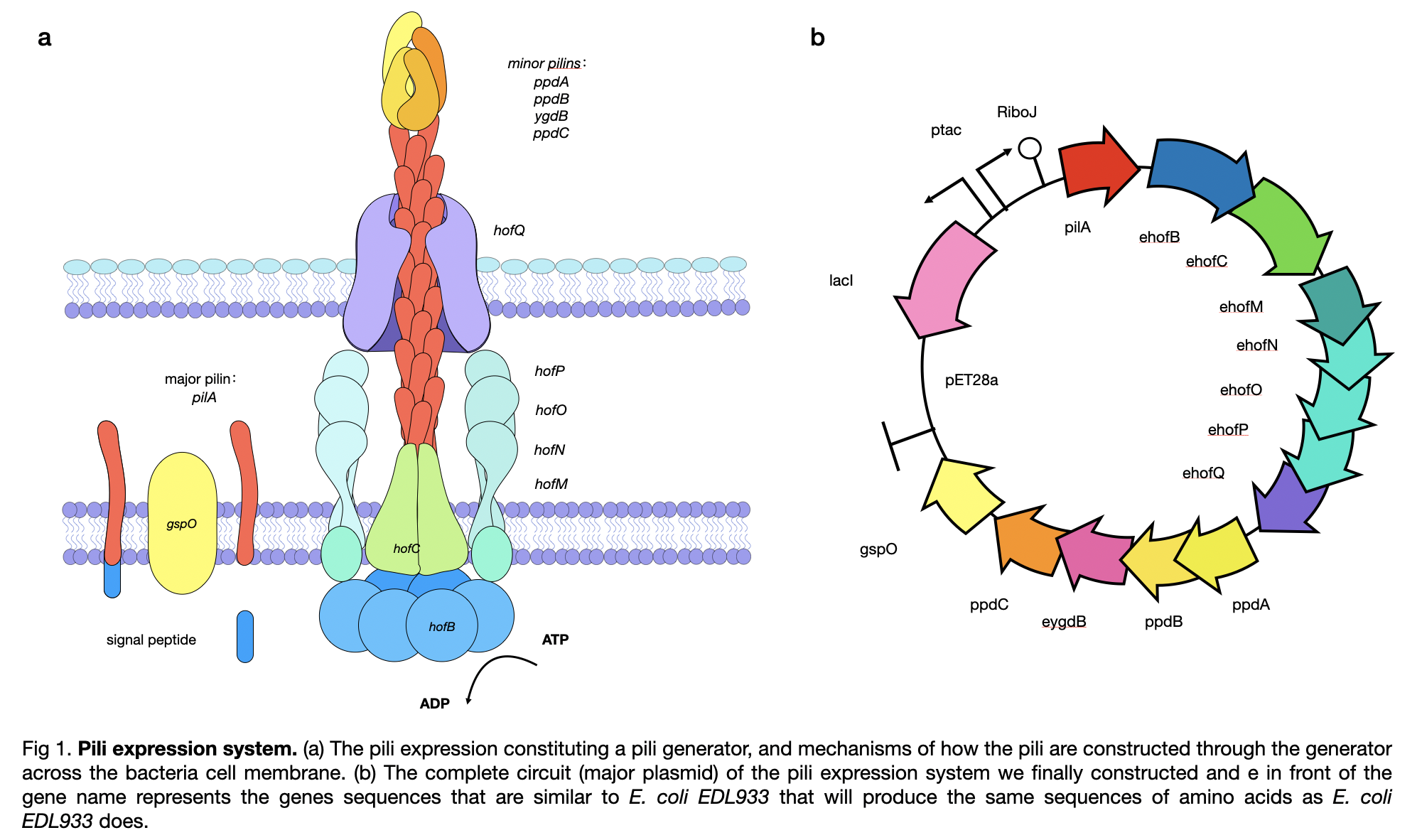
| + | This year, LINKS_China constructed a conductive pilus, GsPilA, through slicing by overlap extension by PCR using 8 different primers to obtain a similar DNA sequence as in the Geobacter sulfurreducens, which produce the same amino acid sequence. To get a complete pili production system, we constructed the generator of the pili and obtained a complete circuit of <partinfo>BBa_K3552010</partinfo> . | ||
| + | |||
| + | [[File:T--LINKS China--Figure pili expression.jpeg|800px]] | ||
| + | |||
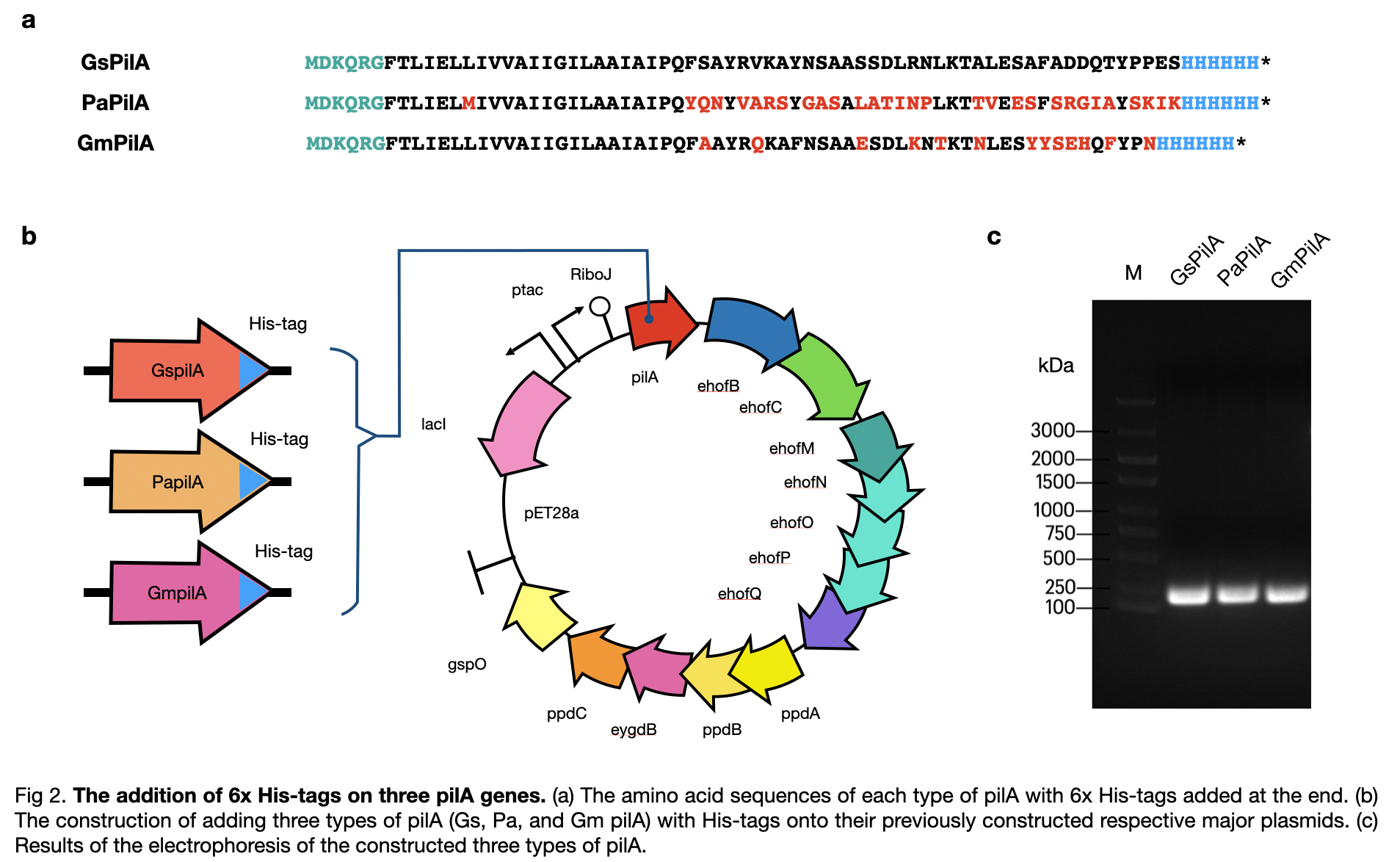
| + | [[File:T--LINKS China--Figure his-tag addition.jpeg|800px]] | ||
| + | |||
| + | For pili expression, we chose E.coli BL21 because it is the best fit for our project as it has the highest pili yield and is easy to obtain. On the cultivation part, we cultured the bacteria in solid M9 mediums. Additionally, we provided the bacteria with glycerol as the carbon source because it was confirmed to help to improve the conductivity of the pili produced. | ||
| + | |||
| + | ===Protein production=== | ||
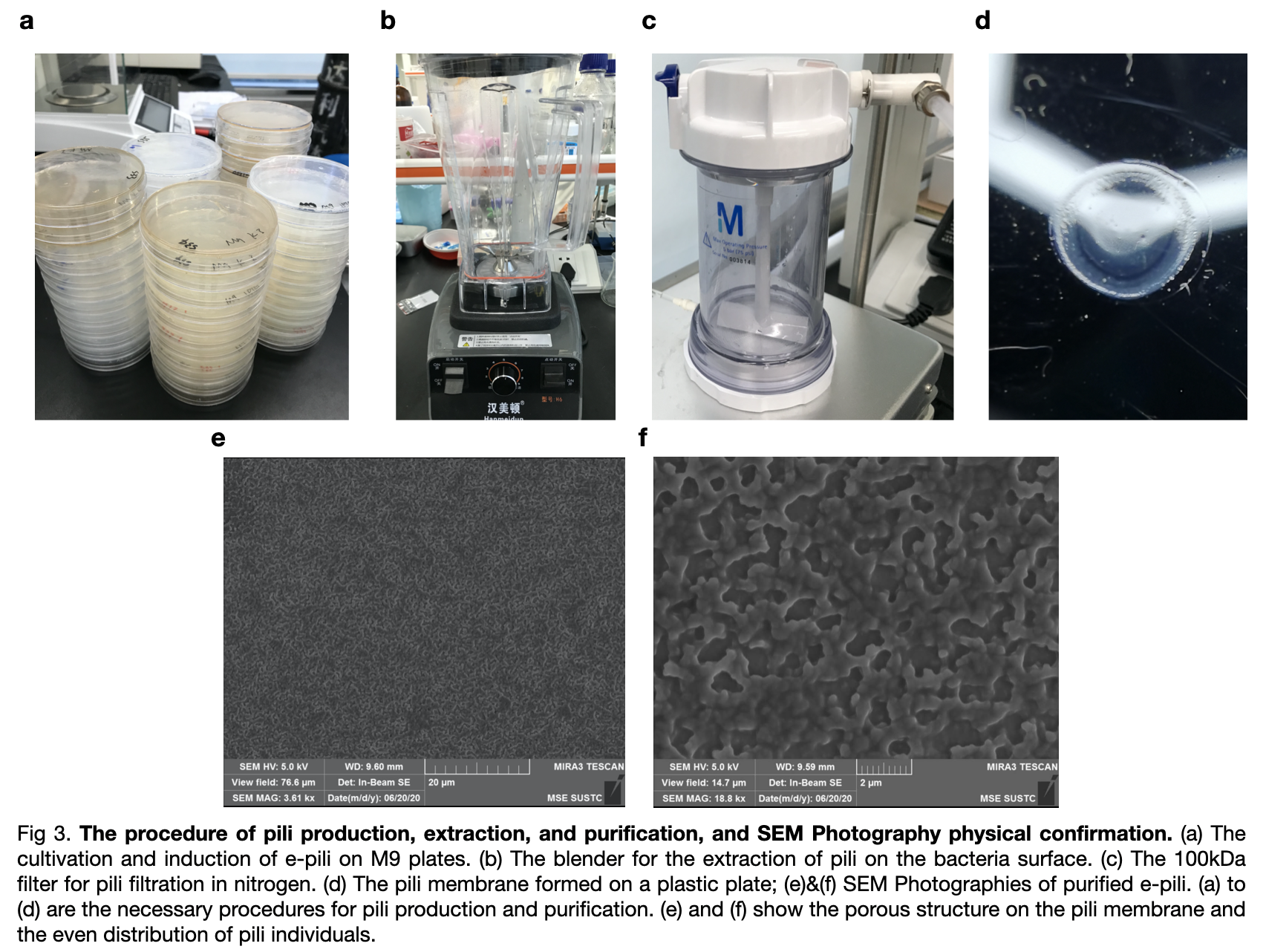
| + | Transformed <partinfo>BBa_K3552010</partinfo> into Escherichia coli(E.coli) BL21, we cultivated the bacteria and harvested the bacteria from M9 plates after 48h cultivation by scrapping them off again with liquid M9 solution and collect the bacteria solution. Finally, by conducting the extraction and purification of the samples, we managed to get an amount Gs pili solution dissolving in 150mM ethanol-amine after filtration with a 100kDa membrane in nitrogen gas. | ||
| + | |||
| + | [[File:T--LINKS China--Figure pilin production.jpeg|800px]] | ||
| + | |||
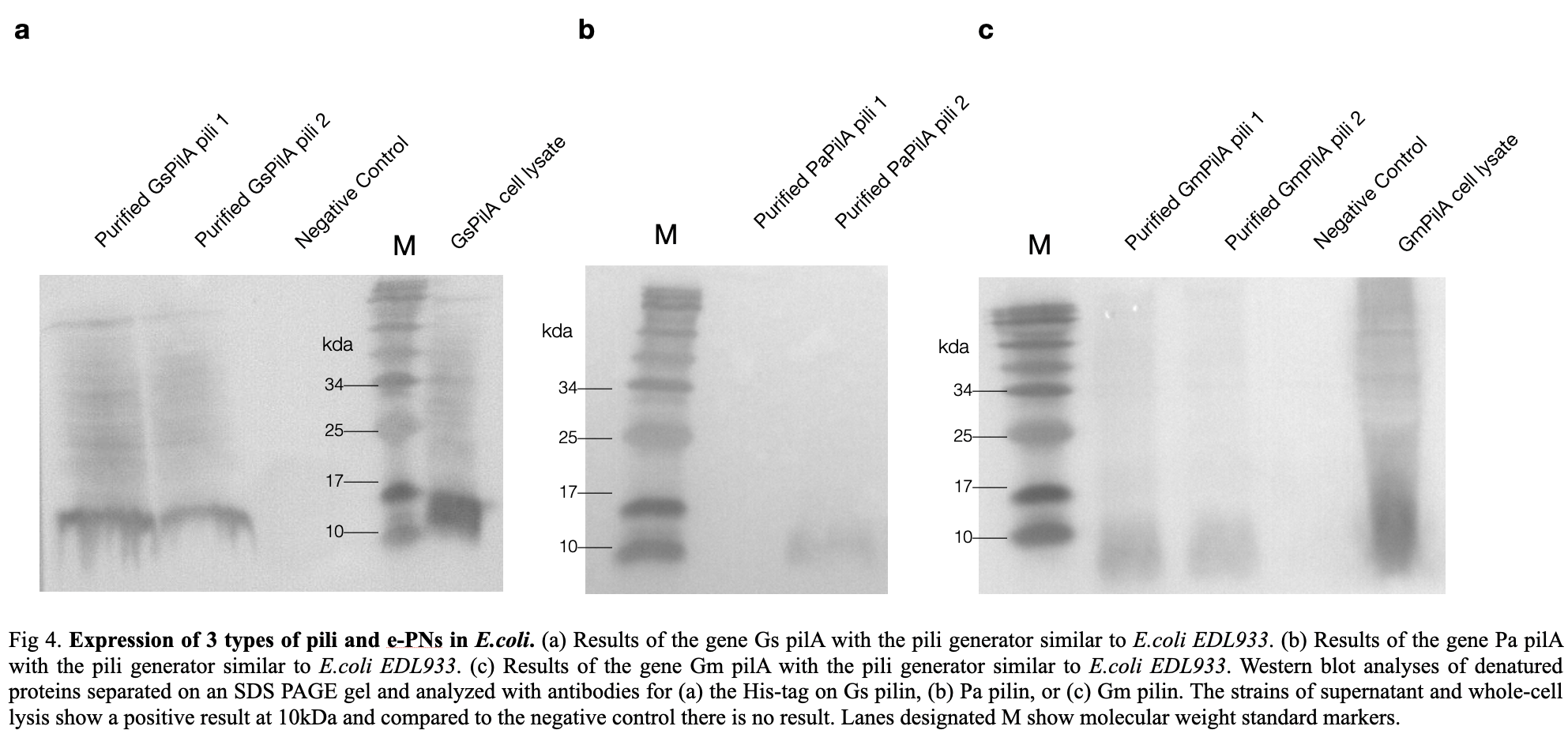
| + | We also ordered an SEM Photography for our pili on the photographs, we can see that the pili were distributed evenly on the surface in porous structures between each pili individuals. To confirm the E.coli produced the correct e-pili, we conducted a Western Blot experiment to convince the production. According to the positive strands on the membrane, we confirmed pili, with an expected molecular mass of 10kDa, was produced and purified successfully. | ||
| + | |||
| + | [[File:T--LINKS China--Figure expression in E.coli.jpeg|800px]] | ||
| − | + | ===Conductiviity measure=== | |
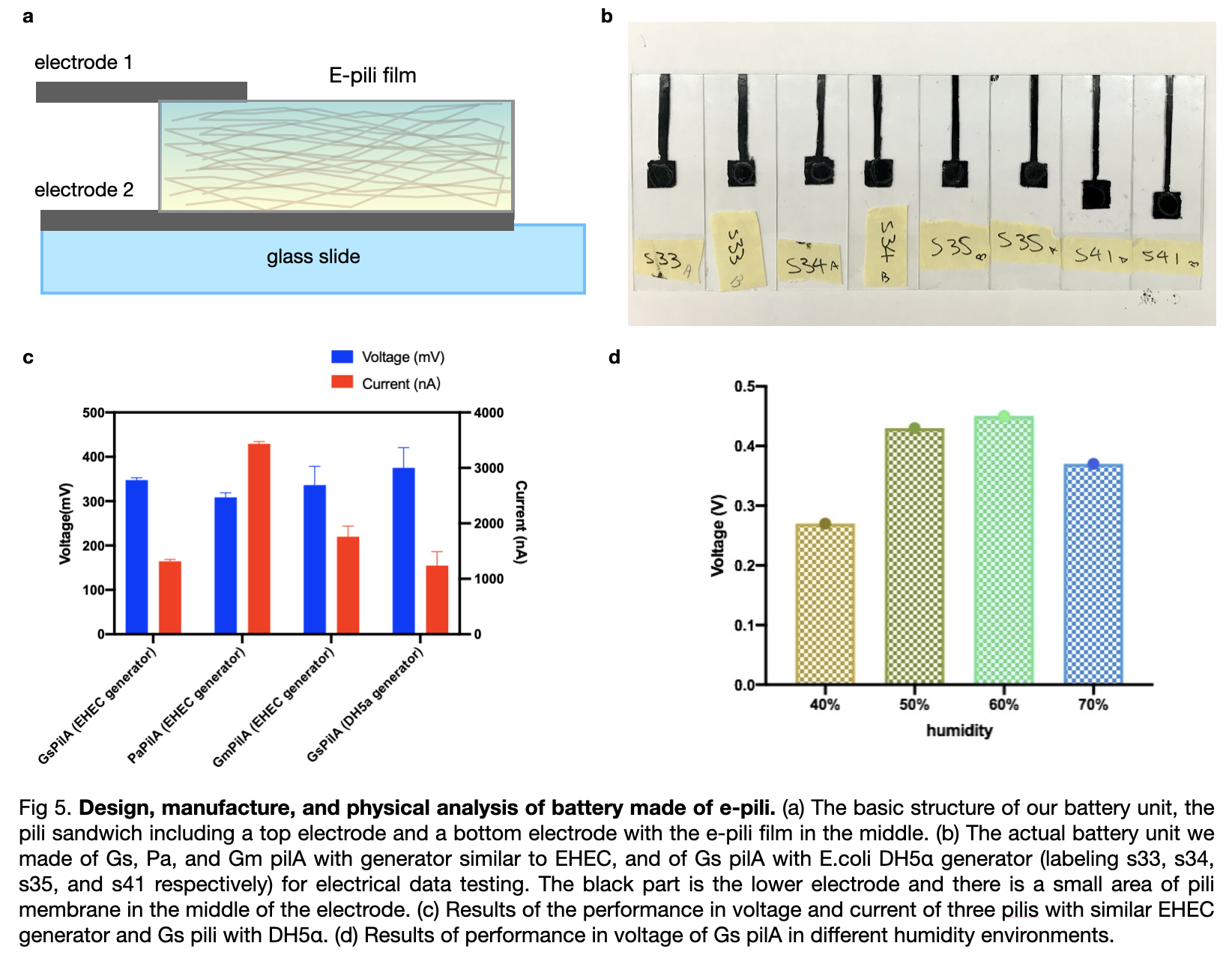
| + | We manufactured the extracted pili into biological batteries and we also conducted experiments to measure the voltage of each battery. First, we manufactured six standard electrodes by using three pili, two pieces each, all with a triple layer of pili covered. Then we measured the voltage and compared the result which showed that Gm pili have the highest value of measured voltage while Gs pili have the least. | ||
| − | + | [[File:T--LINKS China--Figure battery.jpeg|800px]] | |
| − | + | ===New measurement=== | |
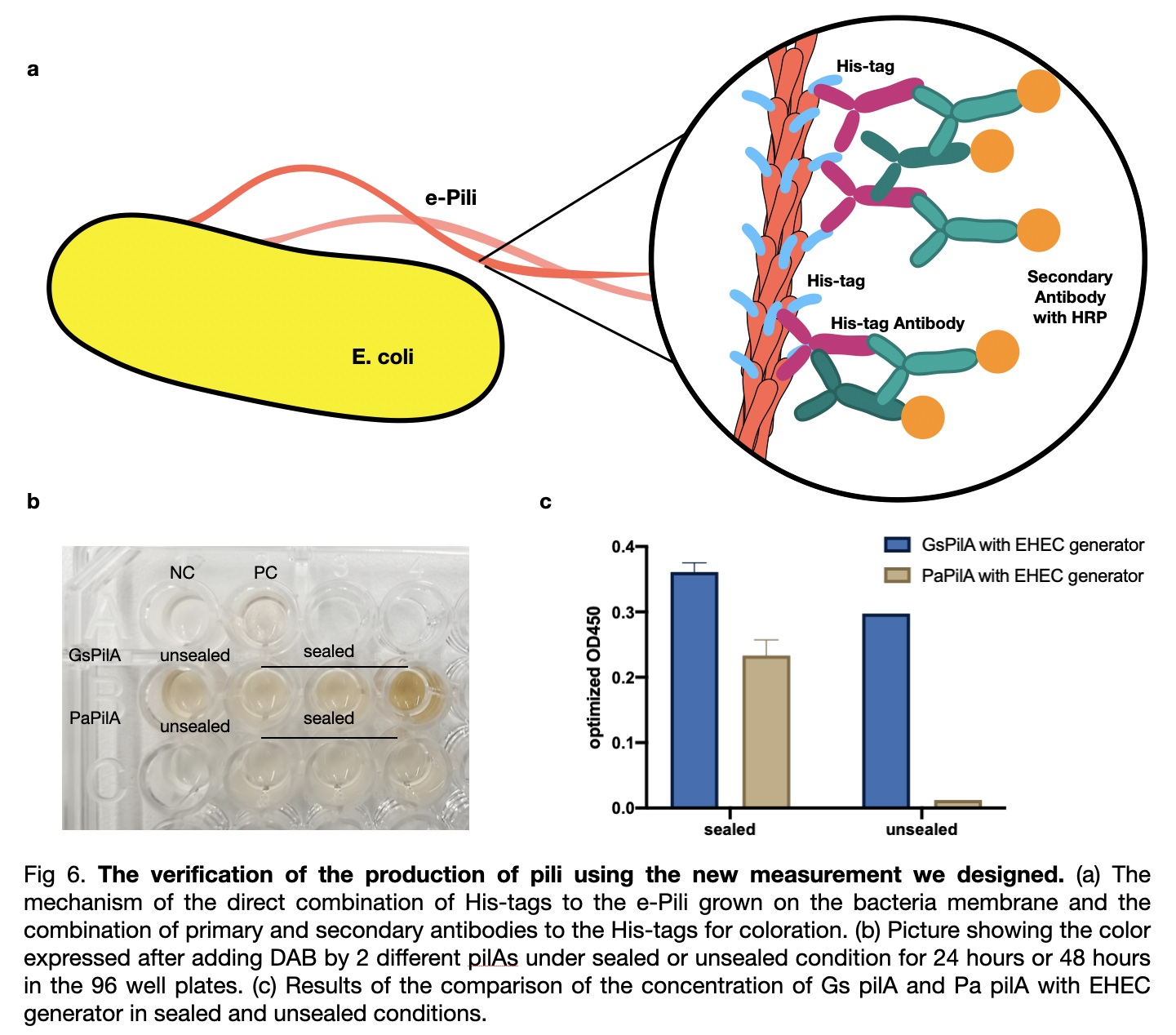
| + | To compare the pili yield, we established a new measurement for a quicker, clearer, and more accurate determination of pili production. We used his-tags antibodies to attach to the his-tags on the pili, and then the secondary antibodies will be attached to the his-tags antibodies for coloration directly on the outer membrane of the bacteria. We analyzed the data and included the effects of two variables on pili production: cultivation time and mobile oxygen presence. | ||
| − | + | [[File:T--LINKS China--Figure verification.jpeg|800px]] | |
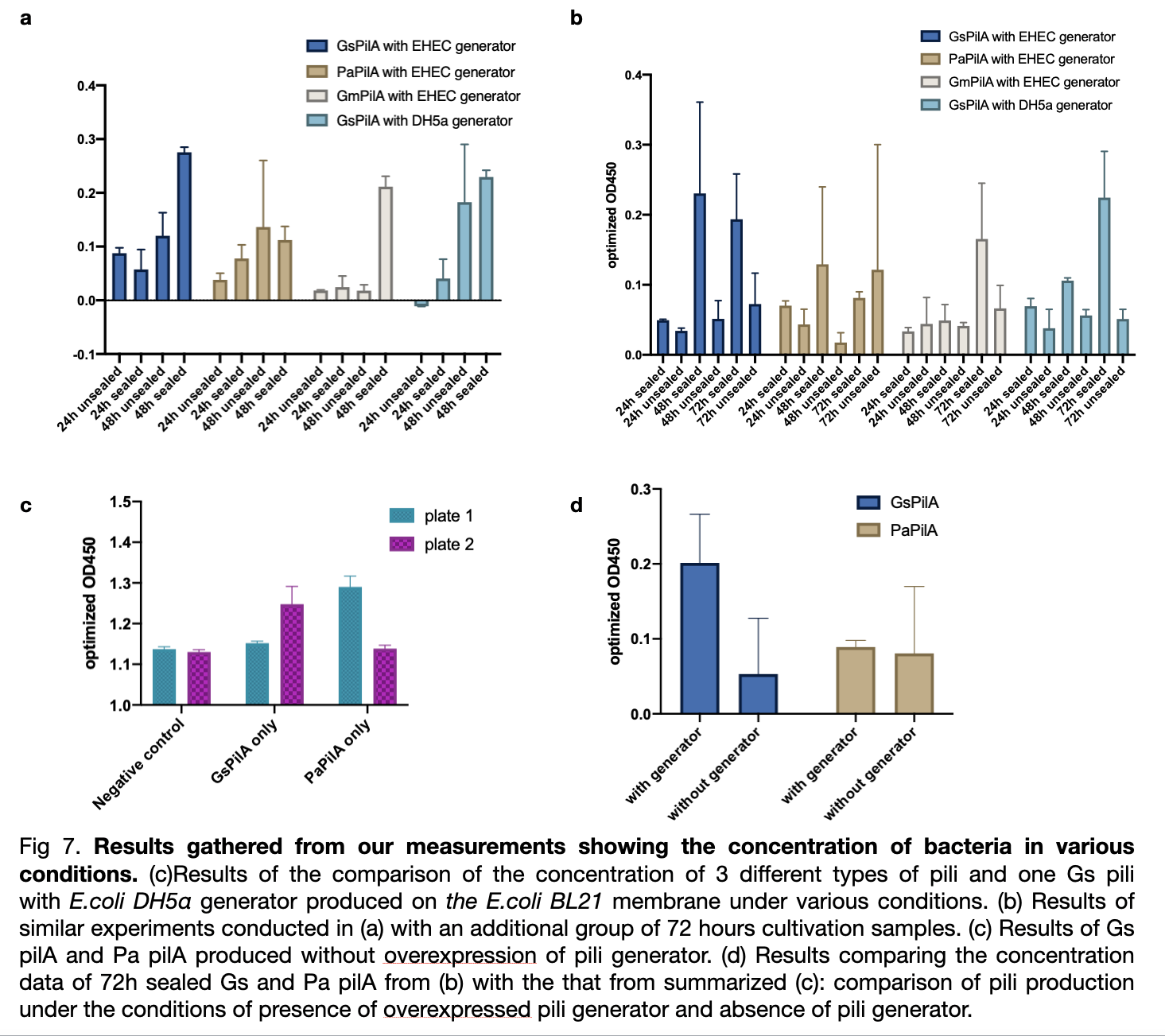
| − | + | [[File:T--LINKS China--Figure results of new measurement.jpeg|800px]] | |
| − | + | ||
| − | + | We measured the absorbance of all samples at od 450 divided by od 600 and subtract the value of negative control to obtain optimized od 450. The result shows a ranking of yield from the highest Gs pili to the lowest Gm pili. The trend for three experiments shows a smaller error bar for expression with EHEC similar generator. The sealed plates also received better results of higher production than the unsealed ones. The optimum cultivation time for GsPilA is 48 hours as it has an optimized od 450 value of 0.4 higher than 72 hours cultivation, whereas at 24 hours cultivation all kinds of bacteria expressed pili poorly. | |
<partinfo>BBa_K3552000 parameters</partinfo> | <partinfo>BBa_K3552000 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
Latest revision as of 23:38, 1 October 2024
GsPilA
Geobacter sulfurreducens pilA(GsPilA) is a type 4 pilus usually found on Geobacter sulfurreducens which can conduct electricity. This part is in the part collection where we provide different conductive pilus based on the same pilin generator. By these different type 4 pilus, we can compare the qualities of stability and yield.
The part collection includes: Parts that are different kinds of type 4 pilus: BBa_K3552000 BBa_K3552001 BBa_K3552002. Parts that are the generator of the type 4 pilus: BBa_K3552003 BBa_K3552004 BBa_K3552005 BBa_K3552006 BBa_K3552007 BBa_K3552008 BBa_K3552018 BBa_K3552019 BBa_K3552020 BBa_K3552021 BBa_K3552022 BBa_K3552023 BBa_K3552024 BBa_K3552025 BBa_K3552026 BBa_K3552027 BBa_K3552028 BBa_K3552029. Parts that are a complete circuit: BBa_K3552009 BBa_K3552010 BBa_K3552011 BBa_K3552012.
Our part collection can instruct other teams to design new rechargeable pilus and substitute them to have more combinations that may achieve better performance in the future when manufacturing the batteries.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Reference
Luna Rico, Areli et al. “Functional reconstitution of the type IVa pilus assembly system from enterohaemorrhagic Escherichia coli.” Molecular microbiology vol. 111,3 (2019): 732-749. doi:10.1111/mmi.14188
Usage and Biology
GsPilA is a fimbrial protein that normally functions in Geobacter sulfurreducens which can conduct electricity. It is a type 4 pili that is long and thin, displaying on the cell surface membrane. It can promote adherence, motility, and transport functions in the bacteria. It is mainly built as helical polymers of a single subunit called the major pilin. These pilins are initially in the plasma membrane and the N-terminal is positively charged. The mature pilins can be extracted through the membrane by the pilin generator.
Structure and function
The pili of Geobacter sulfurreducens are filaments primarily made up of the PilA protein monomer. Due to the short length of the monomer, aromatic residues (tryptophan, phenylalanine, and tyrosine) can be tightly packed along the pili’s length, providing it with conductive properties [1]. This characteristic can be explained by the phenomenon of pi stacking, in which the aromatic side chains align, creating a path for electron transfer to occur [2]. The relationship between the PilA's conductive properties and its protein sequence has further been proven by genetic engineering. For instance, conductivity of pili in which the five aromatic amino acids of the monomers are substituted with alanine is shown to drop to 38 ± 1 μS cm−1, three orders of magnitude lower than the native pili, underscoring the significance of the pi interactions in electron transfer [3]. Furthermore, pH also impacts the conductivity of pili; ranging from 51 mS/cm3 at pH 7 to 37 mS/cm3 at pH 10.5, with the highest conductivity value, 188 mS/cm3, observed at pH 2 [3]. Consistent with this, purified pili films demonstrate non-redox electronic conduction in a buffered aqueous environment; pili can conduct electrons without going through redox processes, which is in line with a metallic charge carrier transport model. This comes in sharp contrast to other organic reactions (e.g. DNA synthesis, photosynthesis), which follow a donor–bridge–acceptor model, where electric potential is passed on by the transfer of electrons from one molecule to the next until it reaches the final acceptor.This also explains how the conductivity of the pili is comparable to synthetic conductive materials, such as carbon nanotubes or silicon nanowires [4] [5].
Novel research and potential applications
Over the last decade, research on pili has revealed a number of potential applications for G. sulfurreducens. For instance, microbial fuel cells can be constructed by connecting aG. sulfurreducens biofilm with the organic waste (e.g. acetate), generating electricity through pili-mediated reduction, [6]. Similarly, the capacity of natural Geobacter to reduce and detoxify radioactive elements and heavy metals in contaminated environments through pili-mediated reductions (e.g soluble Uranium VI to Uranium IV, a form that precipitates and is distributed at a lower rate) can also be enhanced, creating more efficient systems for clearing radiologically and chemically contaminated areas [7] [8]. Electrical biosensors, which could have medical applications in the future for the detection of relevant metabolic compounds, can be created by modifying the C-terminal end of the pilin monomers. Hybrid pili, which consist of wild type, as well as his-tagged and HA-tagged monomers have been shown to conduct electricity better than the wild type pili, as well as change their conductivity potential when binding to the target molecule nickel [9] [10]. Finally, G. sulfurreducens can serve as a model organism for microbially-induced corrosion in metal structures, such as storage tanks and pipes; understanding the electrochemical mechanism of this process can pave the way for technologies that lower maintenance requirements and increase the longevity of these buildings [11].
References (for Structure and function and Novel research and potential applications)
[1] Holmes DE, Dang Y, Walker DJF, Lovley DR. The electrically conductive pili of Geobacter species are a recently evolved feature for extracellular electron transfer. Microbial Genomics. 2016 Aug 25;2(8).
[2] Vargas M, Malvankar NS, Tremblay PL, Leang C, Smith JA, Patel P, et al. Aromatic Amino Acids Required for Pili Conductivity and Long-Range Extracellular Electron Transport in Geobacter sulfurreducens. Giovannoni SJ, editor. mBio. 2013 May;4(2).
[3] Adhikari RY, Malvankar NS, Tuominen MT, Lovley DR. Conductivity of individual Geobacter pili. RSC Advances. 2016 Jan 19;6(10):8354–7.
[4] Ing NL, Nusca TD, Hochbaum AI. Geobacter sulfurreducens pili support ohmic electronic conduction in aqueous solution. Physical Chemistry Chemical Physics 2017 Jan 1;19(32):21791–9.
[5] Ueki T, Walker DJF, Woodard TL, Nevin KP, Nonnenmann SS, Lovley DR. An Escherichia coli Chassis for Production of Electrically Conductive Protein Nanowires. ACS Synthetic Biology. 2020 Mar 3;9(3):647–54.
[6] Nevin KP, Zhang P, Franks AE, Woodard TL, Lovley DR. Anaerobes unleashed: Aerobic fuel cells of Geobacter sulfurreducens. J Power Sources. 2011;196(18):7514–8.
[7] Dang Y, Walker DJF, Vautour KE, Dixon S, Holmes DE. Arsenic Detoxification by Geobacter Species. Liu SJ, editor. Applied and Environmental Microbiology. 2017 Feb 15;83(4).
[8] Cologgi DL, Speers AM, Bullard BA, Kelly SD, Reguera G. Enhanced Uranium Immobilization and Reduction by Geobacter sulfurreducens Biofilms. Parales RE, editor. Applied and Environmental Microbiology. 2014 Nov;80(21):6638–46
[9] Ueki T, Walker DJF, Tremblay PL, Nevin KP, Ward JE, Woodard TL, et al. Decorating the Outer Surface of Microbially Produced Protein Nanowires with Peptides. ACS Synthetic Biology. 2019 Jul 12;8(8):1809–17.
[10] Eric Szmuc, David J.F. Walker, Dmitry Kireev, Deji Akinwande, Derek R. Lovley, Benjamin Keitz, Andrew Ellington. Engineering Geobacter pili to produce metal:organic filaments, Biosensors and Bioelectronics, Volume 222, 2023.
[11] Liu X, Walker DJF, Nonnenmann SS, Sun D, Lovley DR. Direct Observation of Electrically Conductive Pili Emanating from Geobacter sulfurreducens. Papoutsakis ET, editor. mBio. 2021 Aug 31;12(4).
Characterization
Construction
This year, LINKS_China constructed a conductive pilus, GsPilA, through slicing by overlap extension by PCR using 8 different primers to obtain a similar DNA sequence as in the Geobacter sulfurreducens, which produce the same amino acid sequence. To get a complete pili production system, we constructed the generator of the pili and obtained a complete circuit of BBa_K3552010 .
For pili expression, we chose E.coli BL21 because it is the best fit for our project as it has the highest pili yield and is easy to obtain. On the cultivation part, we cultured the bacteria in solid M9 mediums. Additionally, we provided the bacteria with glycerol as the carbon source because it was confirmed to help to improve the conductivity of the pili produced.
Protein production
Transformed BBa_K3552010 into Escherichia coli(E.coli) BL21, we cultivated the bacteria and harvested the bacteria from M9 plates after 48h cultivation by scrapping them off again with liquid M9 solution and collect the bacteria solution. Finally, by conducting the extraction and purification of the samples, we managed to get an amount Gs pili solution dissolving in 150mM ethanol-amine after filtration with a 100kDa membrane in nitrogen gas.
We also ordered an SEM Photography for our pili on the photographs, we can see that the pili were distributed evenly on the surface in porous structures between each pili individuals. To confirm the E.coli produced the correct e-pili, we conducted a Western Blot experiment to convince the production. According to the positive strands on the membrane, we confirmed pili, with an expected molecular mass of 10kDa, was produced and purified successfully.
Conductiviity measure
We manufactured the extracted pili into biological batteries and we also conducted experiments to measure the voltage of each battery. First, we manufactured six standard electrodes by using three pili, two pieces each, all with a triple layer of pili covered. Then we measured the voltage and compared the result which showed that Gm pili have the highest value of measured voltage while Gs pili have the least.
New measurement
To compare the pili yield, we established a new measurement for a quicker, clearer, and more accurate determination of pili production. We used his-tags antibodies to attach to the his-tags on the pili, and then the secondary antibodies will be attached to the his-tags antibodies for coloration directly on the outer membrane of the bacteria. We analyzed the data and included the effects of two variables on pili production: cultivation time and mobile oxygen presence.
We measured the absorbance of all samples at od 450 divided by od 600 and subtract the value of negative control to obtain optimized od 450. The result shows a ranking of yield from the highest Gs pili to the lowest Gm pili. The trend for three experiments shows a smaller error bar for expression with EHEC similar generator. The sealed plates also received better results of higher production than the unsealed ones. The optimum cultivation time for GsPilA is 48 hours as it has an optimized od 450 value of 0.4 higher than 72 hours cultivation, whereas at 24 hours cultivation all kinds of bacteria expressed pili poorly.