Difference between revisions of "Part:BBa K3376012"
| (4 intermediate revisions by the same user not shown) | |||
| Line 109: | Line 109: | ||
= PROOF-OF-CONCEPT = | = PROOF-OF-CONCEPT = | ||
| − | == Antagonism == | + | === Antagonism === |
In the natural environment, S. sanguinis and S. mutans are antagonists which can inhibit the growth of each other. As a result, they are usually used as a model to probe the competition between different species that occupy the same ecological niche. We conducted the experiments of antagonism test between S. mutans and S. sanguinis, S. mutans and WT E. coli Nissle (EcN), S. mutans and GM EcN. The GM EcN has a strong ability to inhibit the growth of S. mutans as shown in the cell shape and size on the agar plates, and even better than S. sanguinis. There’s no antagonistic effect between S. mutans and WT EcN control. The results significantly indicated that the H2O2 produced by the device in GM EcN may be capable of efficiently eliminating S. mutans. | In the natural environment, S. sanguinis and S. mutans are antagonists which can inhibit the growth of each other. As a result, they are usually used as a model to probe the competition between different species that occupy the same ecological niche. We conducted the experiments of antagonism test between S. mutans and S. sanguinis, S. mutans and WT E. coli Nissle (EcN), S. mutans and GM EcN. The GM EcN has a strong ability to inhibit the growth of S. mutans as shown in the cell shape and size on the agar plates, and even better than S. sanguinis. There’s no antagonistic effect between S. mutans and WT EcN control. The results significantly indicated that the H2O2 produced by the device in GM EcN may be capable of efficiently eliminating S. mutans. | ||
| Line 115: | Line 115: | ||
<html> | <html> | ||
<div style="width=100%; display:flex; align-items: center; justify-content: center;"> | <div style="width=100%; display:flex; align-items: center; justify-content: center;"> | ||
| − | <img src="https://static.igem.org/mediawiki/parts/ | + | <img src="https://static.igem.org/mediawiki/parts/6/61/T--Mingdao--vv20.png" style="width:70%;"> |
</div> | </div> | ||
</html> | </html> | ||
| − | == Prototype == | + | === Prototype === |
The handmade lozenge we created is a sweet candy made of 5% sugar alcohols (i.e, xylitol and erythritol) and weighs 1 gram. It contains approximately 3 x 108 CFU (colony forming unit) of EcN (E. coli Nissle) per tablet. To determine the protein expression in our prototype, we made three kinds of lozenges containing GM EcN, which carries the empty vector, the GFP expression device and the AQP-SpxB-KatG expression device. When exposed to blue LED light, EcN with GFP glowed brightly compared to the controls, indicating that GM EcN can express proteins even made into a candy. Moreover, to test the functionality of GM EcN with AQP-SpxB-KatG, we examined the production of H2O2 in the lozenges. The saliva production in the mouth will reach to 3 – 5 ml per minute during eating, chewing or other stimulating activities. Therefore, we dissolved the lozenge in the 3 ml of ddH2O for 5 min and measured H2O2 concentration. GM EcN with AQP-SpxB-KatG can produce 0.58 mM per OD600 compared to the background level (0.21 mM/OD600) by the vector-only control. | The handmade lozenge we created is a sweet candy made of 5% sugar alcohols (i.e, xylitol and erythritol) and weighs 1 gram. It contains approximately 3 x 108 CFU (colony forming unit) of EcN (E. coli Nissle) per tablet. To determine the protein expression in our prototype, we made three kinds of lozenges containing GM EcN, which carries the empty vector, the GFP expression device and the AQP-SpxB-KatG expression device. When exposed to blue LED light, EcN with GFP glowed brightly compared to the controls, indicating that GM EcN can express proteins even made into a candy. Moreover, to test the functionality of GM EcN with AQP-SpxB-KatG, we examined the production of H2O2 in the lozenges. The saliva production in the mouth will reach to 3 – 5 ml per minute during eating, chewing or other stimulating activities. Therefore, we dissolved the lozenge in the 3 ml of ddH2O for 5 min and measured H2O2 concentration. GM EcN with AQP-SpxB-KatG can produce 0.58 mM per OD600 compared to the background level (0.21 mM/OD600) by the vector-only control. | ||
| Line 126: | Line 126: | ||
<html> | <html> | ||
<div style="width=100%; display:flex; align-items: center; justify-content: center;"> | <div style="width=100%; display:flex; align-items: center; justify-content: center;"> | ||
| − | <img src="https://static.igem.org/mediawiki/parts/ | + | <img src="https://static.igem.org/mediawiki/parts/a/a1/T--Mingdao--vv18.gif" style="width:40%;"> |
| + | <img src="https://static.igem.org/mediawiki/parts/9/99/T--Mingdao--vv19.gif" style="width:60%;"> | ||
</div> | </div> | ||
</html> | </html> | ||
In sum, the candy as a prototype we made with GM EcN not only carries live probiotics expressing proteins, but also produces H2O2, implying an effective product against S. mutans for maintenance of oral health. | In sum, the candy as a prototype we made with GM EcN not only carries live probiotics expressing proteins, but also produces H2O2, implying an effective product against S. mutans for maintenance of oral health. | ||
| + | |||
| + | |||
Latest revision as of 07:07, 27 October 2020
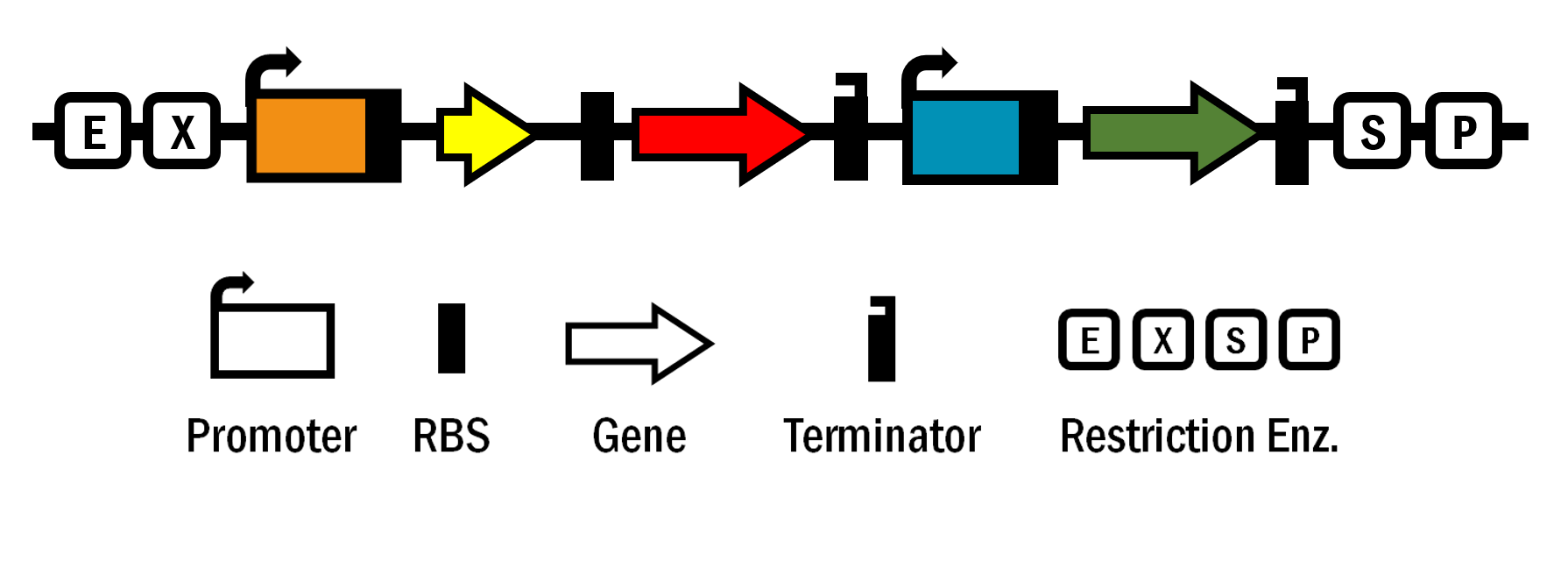
ldhp-AQP-RBS-SpxB-Tr-tpxp-KatG-Tr/pSB1C3
The device contains pyruvate oxidase (SpxB) gene from S. sanguinis to generate H2O2, aquaporin (AQP) gene from S. cristatus to facilitate H2O2 transport, and catalase (KatG) gene from E. coli to revive from oxidative stress by decomposing H2O2 to oxygen and water. The SpxB gene is driven under the constitutive and strong promoter of lactate dehydrogenase (ldhp) of S. mutans. The KatG gene is regulated under the promoter of thiol peroxidase (tpxp) of S. mutans, which is induced by H2O2. Therefore, catalase is expressed in the presence of H2O2 and detoxify the H2O2. Furthermore, the endogenous gene of lactate dehydrogenase is broken to eliminate the production of lactic acid.
THE PROMOTERS
First of all, two kinds of promoters from S. mutans were demonstrated. Lactate dehydrogenase promoter (ldhp) is a strong and constitutive promoter, and thiol peroxidase promoter (tpxp) is induced by the presence of H2O2 in the response to an environmental stress. The gene expression in E. coli Nissle was measured with GFP reporter by the fluorescence intensity at Ex/Em = 483/513 nm. The data showed a strong activity of ldhp and a limited expression of tpxp.


TRANSPORTER AND THE REGULATED PROMOTER
H2O2 permeability across the cell membrane is limited. The aquaporin (AQP) from Streptococcus cristatus facilitates bidirectional transmembrane H2O2 transport. When E. coli Nissle expressing AQP, GFP intensity was enhanced significantly in the presence of AQP and H2O2, indicating AQP functions as H2O2 transporter. The observation is consistent with the study by Huichun Tong, et al.


Furthermore, the GFP intensity was enhanced in response to the increasing concentrations of H2O2 in a dose-dependent manner, suggesting the promoter activity of tpxp was regulated by H2O2.

TOXIN GENERATOR
Pyruvate oxidase (SpxB) of S. sanguinis catalyzes the reaction between pyruvate and oxygen to generate acetyl phosphate and H2O2. It plays a role in antagonistic relationship against S. mutans. The E. coli Nissle carrying SpxB gene is capable of producing 2.15 mM and 1.84 mM of H2O2 per OD600 when grown in 5% and 10% glucose, respectively, compared to the background level of H2O2 production (i.e., 0.33 mM)


TOXIN DETOXIFIER
Catalase (KatG) catalyzes the H2O2 reduction to H2O and O2 and plays a role in E. coli to prevent DNA damage caused by an oxidative stress. The iGEM team Caltech in 2008 created the part of KatG (AHL-inducible KatG expression device, BBa_K137079) and demonstrated the function in minimum inhibitory concentration (MIC) assay, in which the cells were revived from H2O2 shock.
It expected that the bacterial growth was severely influenced in the presence of H2O2. Our data showed that the growth of E. coli Nissle cultured in different concentrations of glucose significantly retarded when expressing AQP and SpxB, further confirming the production of H2O2. In the presence of KatG, the growth rate was increased and comparable with the vector-only control, implied that KatG is capable of decomposing H2O2 and release cells from stress.


H2O2-PRODUCING DEVICE

The lysates of the transformed E. coli Nissle carrying the indicated gene were subjected to SDS-PAGE and Coomassie blue staining. When overexpression under the promoter of ldhp, KatG (80kDa) and AQP (17kDa) have sharp band on the PAGE. However, the predicted 65-kDa of SpxB was not detected, possibly because of the oxidative stress induced by the production of H2O2. In the presence of KatG, SpxB was shown up along with AQP, though expressed at a week level.

The E. coli Nissle carrying the device was cultured in LB broth supplemented with 1%, 5% or 10% glucose. The supernatants of 5-hr culture were subjected to H2O2 production assay. The amounts of H2O2 production were increased dose-dependently in response to the concentration of glucose.

Taken together, based on the results of experiments of H2O2 transportation, H2O2-regulated promoter activity, the growth recovery by catalase, and H2O2 production assay, as well as protein expression analysis, all the data demonstrated the functionality of our composite BioBrick device.
PROOF-OF-CONCEPT
Antagonism
In the natural environment, S. sanguinis and S. mutans are antagonists which can inhibit the growth of each other. As a result, they are usually used as a model to probe the competition between different species that occupy the same ecological niche. We conducted the experiments of antagonism test between S. mutans and S. sanguinis, S. mutans and WT E. coli Nissle (EcN), S. mutans and GM EcN. The GM EcN has a strong ability to inhibit the growth of S. mutans as shown in the cell shape and size on the agar plates, and even better than S. sanguinis. There’s no antagonistic effect between S. mutans and WT EcN control. The results significantly indicated that the H2O2 produced by the device in GM EcN may be capable of efficiently eliminating S. mutans.

Prototype
The handmade lozenge we created is a sweet candy made of 5% sugar alcohols (i.e, xylitol and erythritol) and weighs 1 gram. It contains approximately 3 x 108 CFU (colony forming unit) of EcN (E. coli Nissle) per tablet. To determine the protein expression in our prototype, we made three kinds of lozenges containing GM EcN, which carries the empty vector, the GFP expression device and the AQP-SpxB-KatG expression device. When exposed to blue LED light, EcN with GFP glowed brightly compared to the controls, indicating that GM EcN can express proteins even made into a candy. Moreover, to test the functionality of GM EcN with AQP-SpxB-KatG, we examined the production of H2O2 in the lozenges. The saliva production in the mouth will reach to 3 – 5 ml per minute during eating, chewing or other stimulating activities. Therefore, we dissolved the lozenge in the 3 ml of ddH2O for 5 min and measured H2O2 concentration. GM EcN with AQP-SpxB-KatG can produce 0.58 mM per OD600 compared to the background level (0.21 mM/OD600) by the vector-only control.


In sum, the candy as a prototype we made with GM EcN not only carries live probiotics expressing proteins, but also produces H2O2, implying an effective product against S. mutans for maintenance of oral health.
REFERENCE
- 1. Jessica K Kajfasz, Tridib Ganguly, Emily L Hardin, Jacqueline Abranches, José A Lemos. Transcriptome responses of Streptococcus mutans to peroxide stress: identification of novel antioxidant pathways regulated by Spx. Sci Rep 2017 Nov 22;7(1):16018. doi: 10.1038/s41598-017-16367-5.
- 2. Huichun Tong, Xinhui Wang, Yuzhu Dong, Qingqing Hu, Ziyi Zhao, Yun Zhu, Linxuan Dong, Fan Bai, and Xiuzhu Dong. A Streptococcus aquaporin acts as peroxiporin for efflux of cellular hydrogen peroxide and alleviation of oxidative stress. J Biol Chem. 2019 Mar 22; 294(12): 4583–4595. doi: 10.1074/jbc.RA118.006877
- 3. Lan-yan Zheng, Andreas Itzek, Zhi-yun Chen, and Jens Kreth. Oxygen dependent pyruvate oxidase expression and production in Streptococcus sanguinis. Int J Oral Sci. 2011 Apr; 3(2): 82–89. doi: 10.4248/IJOS11030
- 4. B L Triggs-Raine, B W Doble, M R Mulvey, P A Sorby, and P C Loewen. Nucleotide sequence of katG, encoding catalase HPI of Escherichia coli. J Bacteriol. 1988 Sep; 170(9): 4415–4419. doi: 10.1128/jb.170.9.4415-4419.1988
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 3042
Illegal BglII site found at 3977
Illegal BglII site found at 4104
Illegal BamHI site found at 3894 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 1352
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1271
Illegal SapI site found at 1949
Illegal SapI.rc site found at 2405