Difference between revisions of "Part:BBa K3595008"
Liyingying (Talk | contribs) |
Liyingying (Talk | contribs) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 7: | Line 7: | ||
<!-- Add more about the biology of this part here--> | <!-- Add more about the biology of this part here--> | ||
=Usage and Biology= | =Usage and Biology= | ||
| − | This part can be used as a coding sequence after the promoter pTac and RBS B0034. The feedback inhibition-insensitive SAT can be translated under the induction of IPTG. We constructed plasmids pBR322-KanR-pTac-cysE and pBR322-KanR-pTac-cysE-Mutant,among which the mutants include cysE-256, cysE-5, cysE-11-2, cysE-5-11-2, cysE-256-5,cysE-256-11-2,cysE-256-5-11-2. The constructed plasmid was transformed into <i>Nissle </i> host cell to test its production of cysteine. | + | This part can be used as a coding sequence after the promoter pTac and RBS [[Part:B0034|B0034]]. The feedback inhibition-insensitive SAT can be translated under the induction of IPTG. We constructed plasmids pBR322-KanR-pTac-cysE and pBR322-KanR-pTac-cysE-Mutant,among which the mutants include cysE-256, cysE-5, cysE-11-2, cysE-5-11-2, cysE-256-5,cysE-256-11-2,cysE-256-5-11-2. The constructed plasmid was transformed into <i>Nissle </i> host cell to test its production of cysteine. |
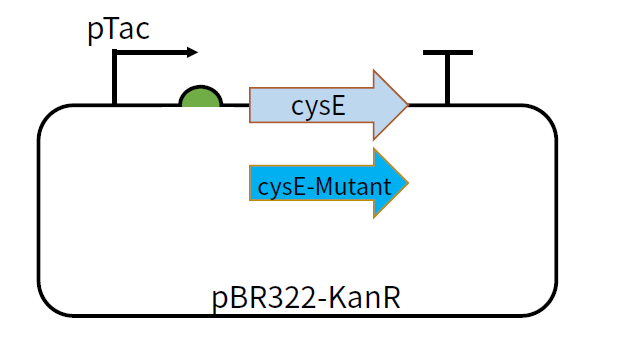
[[File:T--GZ_HFI--cysE.png|600px|thumb|center|The structure of the plasmid pBR322-KanR-pTac-cysE and pBR322-KanR-pTac-cysE-mutant ]] | [[File:T--GZ_HFI--cysE.png|600px|thumb|center|The structure of the plasmid pBR322-KanR-pTac-cysE and pBR322-KanR-pTac-cysE-mutant ]] | ||
==Experimental Setup== | ==Experimental Setup== | ||
| Line 13: | Line 13: | ||
*Plasmid pBR322-KanR-pTac-cysE and pBR322-KanR-pTac-cysE-mutant was transfered into the <i>Nissle </i> host cell,respestively. | *Plasmid pBR322-KanR-pTac-cysE and pBR322-KanR-pTac-cysE-mutant was transfered into the <i>Nissle </i> host cell,respestively. | ||
*Single colonies were selected from the experimental LB-agar plate , then inoculated into test-tube tubes with 4000 μL LB liquid medium with 4uL kanamycin for overnight growth at 37 °C and 200 rpm. | *Single colonies were selected from the experimental LB-agar plate , then inoculated into test-tube tubes with 4000 μL LB liquid medium with 4uL kanamycin for overnight growth at 37 °C and 200 rpm. | ||
| − | *Inoculating 15 uL of culture solution overnight into a 24-well plate containing 3 mL M9 medium for overnight growth at 37 °C and 200 rpm | + | *Inoculating 15 uL of culture solution overnight into a 24-well plate containing 3 mL M9 medium for overnight growth at 37 °C and 200 rpm.The media contained 3 ul kanamycin and 1.5 uL 1M IPTG. At the same time, wild-type Nissle was inoculated as negative control, and M9 medium was used as blank control. |
*Detecting cysteine concentration in culture medium | *Detecting cysteine concentration in culture medium | ||
==Results== | ==Results== | ||
| Line 23: | Line 23: | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K3595008 SequenceAndFeatures</partinfo> | <partinfo>BBa_K3595008 SequenceAndFeatures</partinfo> | ||
| + | |||
| + | <!-- Add more about the biology of this part here--> | ||
| + | =Reference= | ||
| + | Kai Y, et al. Engineering of Escherichia coli L-serine O-acetyltransferase on the basis of crystal structure: desensitization to feedback inhibition by L-cysteine. Protein Eng Des Sel 2006;19(4):163-7. | ||
Latest revision as of 18:59, 27 October 2020
Mutant cysE-5-11-2
cysE-5-11-2 is a mutant of cysE gene, which is obtained by combining the positions of mutations in mutants cysE-5, and cysE-11-2.In the pathway of converting hydrogen sulfide to L-cysteine, L-serine and Acetyl-CoA form O-acetylserine catalyzed by L-serine O-acetyltransferase (SAT). SAT is encoded by gene cysE, and its feedback is inhibited by the final product L-cysteine . In order to induce overproduction of L-cysteine, we need to develop a mutant gene of cysE which will code for feedback inhibition-insensitive SAT.We tried to construct different mutants through site mutation, and cysE-5-11-2 is one of the different mutants.
Usage and Biology
This part can be used as a coding sequence after the promoter pTac and RBS B0034. The feedback inhibition-insensitive SAT can be translated under the induction of IPTG. We constructed plasmids pBR322-KanR-pTac-cysE and pBR322-KanR-pTac-cysE-Mutant,among which the mutants include cysE-256, cysE-5, cysE-11-2, cysE-5-11-2, cysE-256-5,cysE-256-11-2,cysE-256-5-11-2. The constructed plasmid was transformed into Nissle host cell to test its production of cysteine.
Experimental Setup
- Genetic information of cysE,cysE-256, cysE-5, cysE-11-2, cysE-5-11-2, cysE-256-5,cysE-256-11-2,cysE-256-5-11-2 was described on the page ofPart:BBa_K3595004, Part:BBa_K3595005,Part:BBa_K3595006,Part:BBa_K3595007,Part:BBa_K3595009, Part:BBa_K35950010, Part:BBa_K3595011,respectively.
- Plasmid pBR322-KanR-pTac-cysE and pBR322-KanR-pTac-cysE-mutant was transfered into the Nissle host cell,respestively.
- Single colonies were selected from the experimental LB-agar plate , then inoculated into test-tube tubes with 4000 μL LB liquid medium with 4uL kanamycin for overnight growth at 37 °C and 200 rpm.
- Inoculating 15 uL of culture solution overnight into a 24-well plate containing 3 mL M9 medium for overnight growth at 37 °C and 200 rpm.The media contained 3 ul kanamycin and 1.5 uL 1M IPTG. At the same time, wild-type Nissle was inoculated as negative control, and M9 medium was used as blank control.
- Detecting cysteine concentration in culture medium
Results
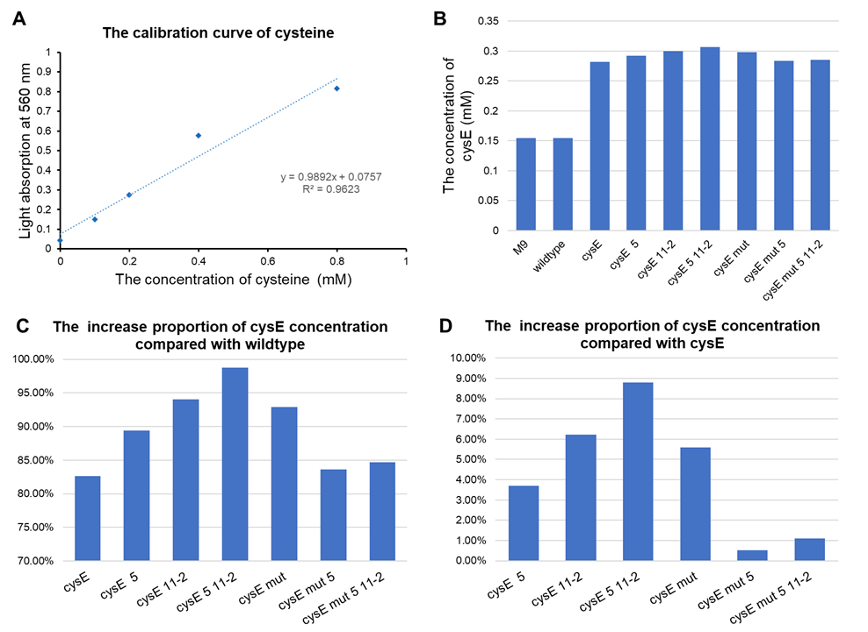
- All of our engineered bacteria have shown great improvement in the ability to produce cysteine, which represents that they can absorb H2S to a larger extent.
- EcN with plasmid pTYT-cysE-5-11-2 had the best effect, 98.72% batter than EcN wildtype and 8.79% better than overexpression of cysE (pTYT-cysE)
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 684
- 1000COMPATIBLE WITH RFC[1000]
Reference
Kai Y, et al. Engineering of Escherichia coli L-serine O-acetyltransferase on the basis of crystal structure: desensitization to feedback inhibition by L-cysteine. Protein Eng Des Sel 2006;19(4):163-7.