Difference between revisions of "Part:BBa K3520018"
| (8 intermediate revisions by 3 users not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K3520018 short</partinfo> | <partinfo>BBa_K3520018 short</partinfo> | ||
| − | + | <partinfo>BBa_K3520018 SequenceAndFeatures</partinfo> | |
| + | <br><br> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | ==Description== | ||
| + | This composite part consists of the Transcriptional Units BBa_K3520017(Signal peptide+Reflectin) and BBa_K3520020(GFP superfolder) of the Transcriptional Units where we used the promoter, RBS and terminator, (BBa_K3520007, BBa_K3520008 and BBa_K1172903) to ensure its successful translation in <i>Flavobacterium johnsoniae</i>. The same procedure was followed for sfGFP, but without the signal peptide, as it was not needed. Those genes are incorporated into the pHimarEm1 transposon that is found in pHimarEm1 plasmid, using Type IIS assembly (level 1) standard. The plasmid containing transcriptional units (GFP and reflectin) is inserted with conjugation in the flavobacteria using <i>E. coli S17-1</i> as the donor of plasmid. After conjugation occurs, the two transcriptional units are transported into the Flavobacterium species genome. | ||
| + | |||
| + | |||
| + | |||
| + | ==Plasmid map== | ||
| + | |||
| + | [[File:T--Athens--phimarem1_gfp_reflectin_plasmid_map.png|800px|thumb|center|Figure 1: pHimarEm1 with superfolderGFP and reflectin insert.]] | ||
| + | |||
| + | ==iGEM KU Istanbul 2020== | ||
| + | <br /><br /> | ||
| + | This part was designed during the Partnership of iGEM Athens 2020 and iGEM KU Istanbul 2020. | ||
| + | The latter team is creating a communication scheme between humans and biological cells by morphing cells into lasers. By this technology, they will be able to detect changes inside and around cells and tissues. These cell lasers can be employed in diagnostics and therapeutic purposes alongside as a high throughput method in basic research. | ||
| + | |||
| + | ==iGEM Athens 2020== | ||
| + | <br /><br /> | ||
| + | iGEM Athens 2020 team during the project MORPHÆ works with Flavobacteriia to produce a non-cellular structurally coloured biomaterial which will require the secretion of a biomolecule that Flavobacteriia do not normally secrete. Our hypothesis is that the formed matrix will have a structure similar to that of the biofilm and thus, it will provide the material with macroscopically the same colouration properties as the biofilm.<br /><br /> | ||
| + | |||
| + | So these two teams above, collaborated in a creative way and iGEM Athens designed a cloning experiment in which Flavobacteriia will express reflectin with a signal peptide which will translocate it to the outer membrane and GFP superfolde. As a result, the biolaser designed by iGEM KU Istanbul will be able to track genetically modified Flavobacteriia. | ||
| + | |||
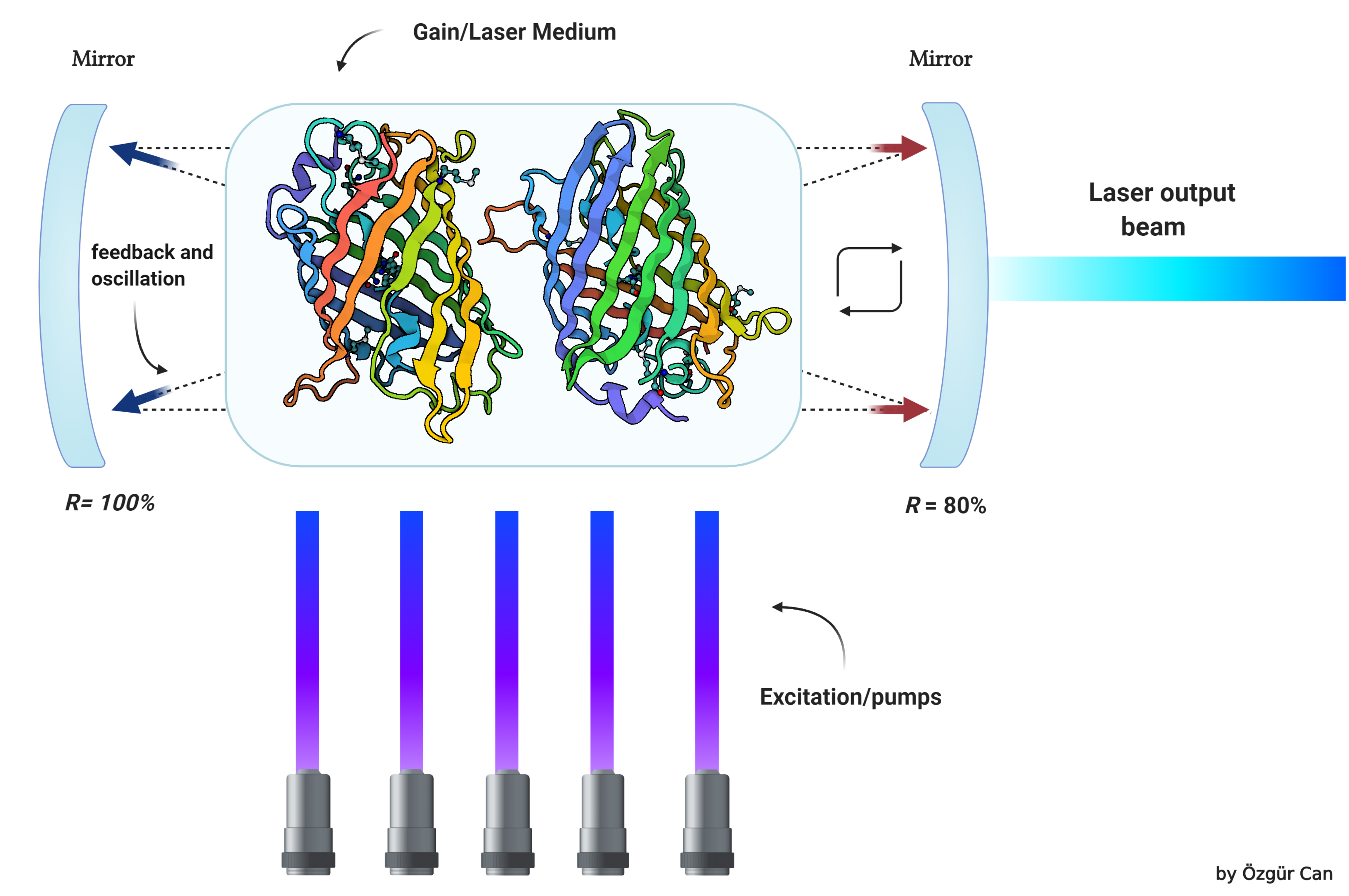
| + | [[File:T--Athens--Instanbul_Collaboration_Biolaser.png|800px|thumb|center|Figure 2: Basics of a biolaser]] | ||
| − | + | ==Further information about Biolaser's Function== | |
| − | == | + | <br /><br /> |
| − | < | + | Biological laser or biolaser is an emerging concept with huge potential in biological and biomedical research. Biolaser is a new type of laser which has biological materials as part of its gain medium or optical feedback. Biolasers are very close to fluorescence microscopy where active molecules (fluorescent) emit light upon excitation by an external laser. However, biolasers are different from fluorescence with respect to optical feedback and gain. The light emitted from a biolaser is coherent, meaning it provides a high signal to noise ratio, consists of a narrow spectrum, and can focus on a small spot, whereas fluorescence radiation is dispersive and very weak compared to biolasers. We are developing a biological laser concept in which no artificial cavities will be used to confine light as opposed to conventional biolasers. Cavities we will be using are composed of natural biological materials which cover the membrane of the cell. So the biolaser concept is fully composed of biological materials. This goal will be achieved by genetically modifying cells to express silicatein and reflectin proteins as well as fluorescent proteins. Expressed silicatein and reflectins will be transported toward the membrane of the cell, and these proteins will be covering the inside and/or outside of the cell. |
| − | + | ||
Latest revision as of 03:46, 28 October 2020
Recombinant pHimarEm1 with Reflectin/signalpeptide/GFP TU
- 10INCOMPATIBLE WITH RFC[10]Illegal prefix found in sequence at 6663
Illegal suffix found in sequence at 1 - 12INCOMPATIBLE WITH RFC[12]Illegal EcoRI site found at 6663
Illegal NheI site found at 330
Illegal SpeI site found at 2
Illegal PstI site found at 16
Illegal NotI site found at 9
Illegal NotI site found at 6669 - 21INCOMPATIBLE WITH RFC[21]Illegal EcoRI site found at 6663
Illegal BglII site found at 652
Illegal BglII site found at 3191
Illegal BglII site found at 3850
Illegal XhoI site found at 287
Illegal XhoI site found at 3488 - 23INCOMPATIBLE WITH RFC[23]Illegal prefix found in sequence at 6663
Illegal suffix found in sequence at 2 - 25INCOMPATIBLE WITH RFC[25]Illegal prefix found in sequence at 6663
Illegal XbaI site found at 6678
Illegal SpeI site found at 2
Illegal PstI site found at 16
Illegal NgoMIV site found at 1316
Illegal AgeI site found at 2883 - 1000COMPATIBLE WITH RFC[1000]
Description
This composite part consists of the Transcriptional Units BBa_K3520017(Signal peptide+Reflectin) and BBa_K3520020(GFP superfolder) of the Transcriptional Units where we used the promoter, RBS and terminator, (BBa_K3520007, BBa_K3520008 and BBa_K1172903) to ensure its successful translation in Flavobacterium johnsoniae. The same procedure was followed for sfGFP, but without the signal peptide, as it was not needed. Those genes are incorporated into the pHimarEm1 transposon that is found in pHimarEm1 plasmid, using Type IIS assembly (level 1) standard. The plasmid containing transcriptional units (GFP and reflectin) is inserted with conjugation in the flavobacteria using E. coli S17-1 as the donor of plasmid. After conjugation occurs, the two transcriptional units are transported into the Flavobacterium species genome.
Plasmid map
iGEM KU Istanbul 2020
This part was designed during the Partnership of iGEM Athens 2020 and iGEM KU Istanbul 2020.
The latter team is creating a communication scheme between humans and biological cells by morphing cells into lasers. By this technology, they will be able to detect changes inside and around cells and tissues. These cell lasers can be employed in diagnostics and therapeutic purposes alongside as a high throughput method in basic research.
iGEM Athens 2020
iGEM Athens 2020 team during the project MORPHÆ works with Flavobacteriia to produce a non-cellular structurally coloured biomaterial which will require the secretion of a biomolecule that Flavobacteriia do not normally secrete. Our hypothesis is that the formed matrix will have a structure similar to that of the biofilm and thus, it will provide the material with macroscopically the same colouration properties as the biofilm.
So these two teams above, collaborated in a creative way and iGEM Athens designed a cloning experiment in which Flavobacteriia will express reflectin with a signal peptide which will translocate it to the outer membrane and GFP superfolde. As a result, the biolaser designed by iGEM KU Istanbul will be able to track genetically modified Flavobacteriia.
Further information about Biolaser's Function
Biological laser or biolaser is an emerging concept with huge potential in biological and biomedical research. Biolaser is a new type of laser which has biological materials as part of its gain medium or optical feedback. Biolasers are very close to fluorescence microscopy where active molecules (fluorescent) emit light upon excitation by an external laser. However, biolasers are different from fluorescence with respect to optical feedback and gain. The light emitted from a biolaser is coherent, meaning it provides a high signal to noise ratio, consists of a narrow spectrum, and can focus on a small spot, whereas fluorescence radiation is dispersive and very weak compared to biolasers. We are developing a biological laser concept in which no artificial cavities will be used to confine light as opposed to conventional biolasers. Cavities we will be using are composed of natural biological materials which cover the membrane of the cell. So the biolaser concept is fully composed of biological materials. This goal will be achieved by genetically modifying cells to express silicatein and reflectin proteins as well as fluorescent proteins. Expressed silicatein and reflectins will be transported toward the membrane of the cell, and these proteins will be covering the inside and/or outside of the cell.