Difference between revisions of "Part:BBa K3457015"
| (3 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K3457015 short</partinfo> | <partinfo>BBa_K3457015 short</partinfo> | ||
| − | + | <p> This biological part is the CDS sequence of Super-folder Green Fluorescent Protein, also called sfGFP. It was mainly used as the control group of our project this year. We also use it to test J23109, J23107 and J23100 promoters. </p> | |
| + | |||
| + | ===Contribution=== | ||
| + | <h2><b>Group: QHFZ-China iGEM 2020</b></h2> | ||
| + | <h3><b>Author: Yixian Yang</b></h3> | ||
| + | <h3><b>Design</b></h3> | ||
| + | [[File:T--QHFZ-China--sfGFP basic design.png|400px|thumb|left|Figure 1. The Schematic cartoon of the DNA construct of BBa_K3457015.]] | ||
| + | |||
| + | <p style="clear:left;"> This part is the CDS of sfGFP. The protein is widely used. With the help of a company, we optimized the sequence, so that the part is suitable to work in <i>E. coli</i> and there is no biobrick restriction enzyme cutting site in it. Moreover, we added an 6x His tag at the N-terminal of sfGFP, so it can be easily detected by Western Blot (Fig.1). This year, the part is used in the composite part [https://parts.igem.org/Part:BBa_K3457048 BBa_K3457048], [https://parts.igem.org/Part:BBa_K3457037 BBa_K3457037], [https://parts.igem.org/Part:BBa_K3457051 BBa_K3457051], [https://parts.igem.org/Part:BBa_K3457054 BBa_K3457054].</p> | ||
| + | |||
| + | <h3><b>Documentation:</b></h3> | ||
| + | <h4><b>Introduction: </b></h4> | ||
| + | <p style="clear:left;"> This year, we tried to introduce a new biopreservation method. We used freeze-drying to make the engineered into dry powder. Then the powder can be stored at room temperature for a long time. This method can make the storage of bacteria get rid of ultra-low temperature freezer, so that it will promote the practical application of engineered bacteria out of laboratory. However, the stresses during freeze-drying and subsequent dry storage, including freeze, dry and vacuum, are lethal to bacteria. We use TDPs, to help bacteria survive the situation. In these tests, sfGFP was the control group.</p> | ||
| + | |||
| + | <h4><b>Protocol: </b></h4> | ||
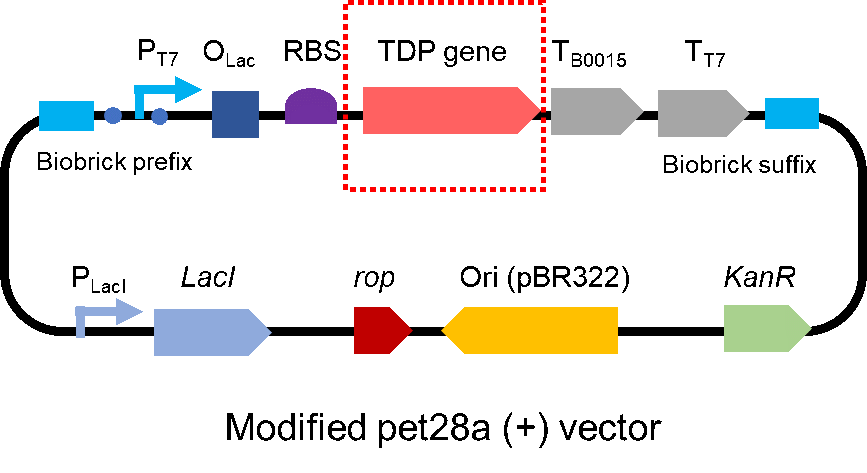
| + | <p style="clear:left;"> We modified a frequently and widely used vector, pet28a+ and put this part into it (Fig. 2). Then we transformed the plasmid into <i>E. coli</i> BL21 strain. </p> | ||
| + | |||
| + | [[File:T--QHFZ-China--pet28am.png|400px|thumb|left|Figure 2. The Schematic cartoon of the vector.]] | ||
| + | |||
| + | <p style="clear:left;"> In general, we used the following protocol to detect fluorescence:<br> | ||
| + | (1) Pick clones which are in good condition and put them into 500 μL LB medium containing antibiotics. Shake them to grow at 37℃ for 5~7 hours until the bacteria solution becomes turbid. <br> | ||
| + | (2) Add 2mM iPTG into 3 mL LB medium containing antibiotics. Add 3 μL of the bacteria solution mentioned in step 1 to dilute the bacteria by the ratio of 1:1000. Shake the solution to grow the bacteria at 37℃ overnight.<br> | ||
| + | (3) 100 μL bacteria solution was put into a well of a 96-well palte. The GFP fluorescence and OD<sub>600</sub> were detected by a microplate readers (Bio-Teck). The parameters are: exciting light: 488 nm, light reception: 520 nm, gain: 50. <br> | ||
| + | (4) The value of LB medium was deducted from the result above. GFP / OD<sub>600</sub> was calculated.<br> | ||
| + | However, to the precise experiment. step (3) was a little modified. The bacteria solution was centrifuged and the LB medium was removed. Then the bacteria was resuspended by PBS. The GFP fluorescence and OD<sub>600</sub> of PBS both almost equalled to 0. | ||
| + | </p> | ||
| + | |||
| + | <h4><b>Results:</b></h4> | ||
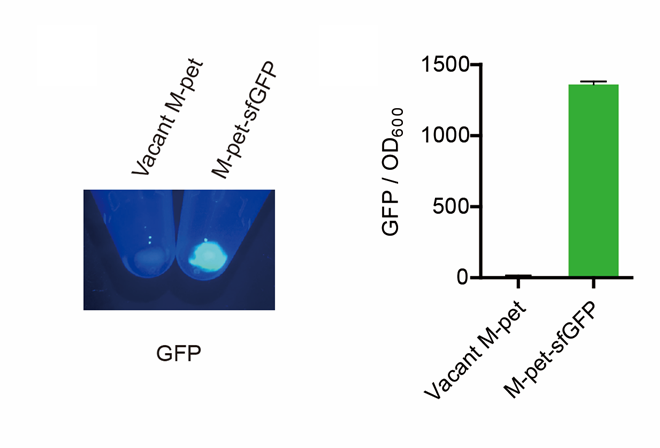
| + | <p style="clear:left;"> In the modified pet28a vector, sfGFP was normally expressed.</p> | ||
| + | |||
| + | [[File:T--QHFZ-China--pet28am sfGFP.png|400px|thumb|left|Figure 3. sfGFP was expressed with T7 promoter.]] | ||
| + | |||
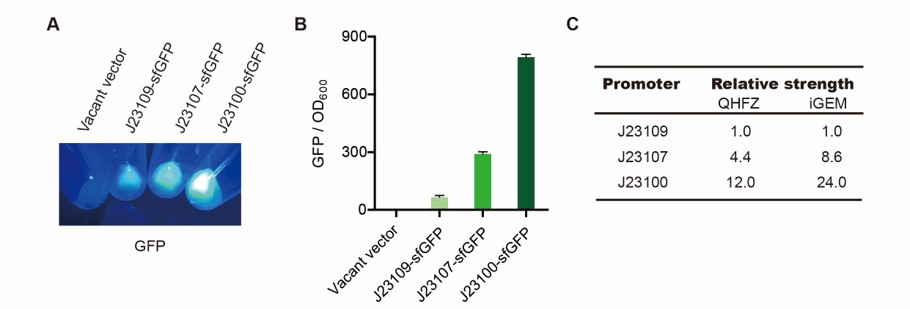
| + | <p style="clear:left;"> The we changed the T7 promoter into J23100, J23107 and J23109 promoters, and tested the fluorescence (Fig. 7). </p> | ||
| + | |||
| + | [[File:T--QHFZ-China--sfGFP.jpg|400px|thumb|left|Figure 4. sfGFP was expressed with other promoters.]] | ||
| + | |||
| + | <p style="clear:left;"> </p> | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Latest revision as of 00:39, 27 October 2020
sfGFP with a 6X His tag
This biological part is the CDS sequence of Super-folder Green Fluorescent Protein, also called sfGFP. It was mainly used as the control group of our project this year. We also use it to test J23109, J23107 and J23100 promoters.
Contribution
Group: QHFZ-China iGEM 2020
Author: Yixian Yang
Design
This part is the CDS of sfGFP. The protein is widely used. With the help of a company, we optimized the sequence, so that the part is suitable to work in E. coli and there is no biobrick restriction enzyme cutting site in it. Moreover, we added an 6x His tag at the N-terminal of sfGFP, so it can be easily detected by Western Blot (Fig.1). This year, the part is used in the composite part BBa_K3457048, BBa_K3457037, BBa_K3457051, BBa_K3457054.
Documentation:
Introduction:
This year, we tried to introduce a new biopreservation method. We used freeze-drying to make the engineered into dry powder. Then the powder can be stored at room temperature for a long time. This method can make the storage of bacteria get rid of ultra-low temperature freezer, so that it will promote the practical application of engineered bacteria out of laboratory. However, the stresses during freeze-drying and subsequent dry storage, including freeze, dry and vacuum, are lethal to bacteria. We use TDPs, to help bacteria survive the situation. In these tests, sfGFP was the control group.
Protocol:
We modified a frequently and widely used vector, pet28a+ and put this part into it (Fig. 2). Then we transformed the plasmid into E. coli BL21 strain.
In general, we used the following protocol to detect fluorescence:
(1) Pick clones which are in good condition and put them into 500 μL LB medium containing antibiotics. Shake them to grow at 37℃ for 5~7 hours until the bacteria solution becomes turbid.
(2) Add 2mM iPTG into 3 mL LB medium containing antibiotics. Add 3 μL of the bacteria solution mentioned in step 1 to dilute the bacteria by the ratio of 1:1000. Shake the solution to grow the bacteria at 37℃ overnight.
(3) 100 μL bacteria solution was put into a well of a 96-well palte. The GFP fluorescence and OD600 were detected by a microplate readers (Bio-Teck). The parameters are: exciting light: 488 nm, light reception: 520 nm, gain: 50.
(4) The value of LB medium was deducted from the result above. GFP / OD600 was calculated.
However, to the precise experiment. step (3) was a little modified. The bacteria solution was centrifuged and the LB medium was removed. Then the bacteria was resuspended by PBS. The GFP fluorescence and OD600 of PBS both almost equalled to 0.
Results:
In the modified pet28a vector, sfGFP was normally expressed.
The we changed the T7 promoter into J23100, J23107 and J23109 promoters, and tested the fluorescence (Fig. 7).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]