Difference between revisions of "Part:BBa K117002"
m |
|||
| (12 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| + | <html> | ||
| + | <div id="arrow"> | ||
| + | <a href="http://2008.igem.org/Team:NTU-Singapore"> | ||
| + | <img src="https://static.igem.org/mediawiki/parts/2/26/Back_to_ntu_copy.png" | ||
| + | alt="Back to NTU@iGEM" | ||
| + | title="Back to NTU@iGEM"> | ||
| + | </a> | ||
| + | </div> | ||
| + | </html> | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K117002 short</partinfo> | <partinfo>BBa_K117002 short</partinfo> | ||
| Line 5: | Line 14: | ||
The integral part of the AI-2 quorum-sensing (QS) system consists of a transporter complex, LsrABCD; its repressor, LsrR; and a cognate signal kinase, LsrK. | The integral part of the AI-2 quorum-sensing (QS) system consists of a transporter complex, LsrABCD; its repressor, LsrR; and a cognate signal kinase, LsrK. | ||
Once uptaken into the cell, AI-2 will be phosphorylated under the effect of LsrK. Without the presence of AI-2, LsrA promoter is inhibited by the repressor, LsrR. Phosphorylated AI-2 will prevent LsrR from inhibiting this promoter, hence activating it so that the downstream sequence can be expressed. | Once uptaken into the cell, AI-2 will be phosphorylated under the effect of LsrK. Without the presence of AI-2, LsrA promoter is inhibited by the repressor, LsrR. Phosphorylated AI-2 will prevent LsrR from inhibiting this promoter, hence activating it so that the downstream sequence can be expressed. | ||
| − | To prevent the cell from self-inducing this promoter, we must suppress its AI-2 producing capability. It can be done by knocking out LuxS, one of the vital gene required for producing AI-2. By doing that, LsrA promoter can only be activated under the presence of AI-2 from other sources than the host cell which is LuxS mutant (say, by contact with normal cells). | + | To prevent the cell from self-inducing this promoter, we must suppress its AI-2 producing capability. It can be done by knocking out LuxS, one of the vital gene required for producing AI-2. By doing that, LsrA promoter can only be activated under the presence of AI-2 from other sources than the host cell which is LuxS mutant (say, by contact with normal cells). |
[[Image:LsrA.png|thumb|center|700px| Effects of AI-2 on LsrA promoter]] | [[Image:LsrA.png|thumb|center|700px| Effects of AI-2 on LsrA promoter]] | ||
More information on AI-2 quorum-sensing (QS) system please refer to: | More information on AI-2 quorum-sensing (QS) system please refer to: | ||
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1952038 | http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1952038 | ||
| + | |||
| + | ==Modelling The Lsr Promoter - Contribution by Manchester iGEM 2021 == | ||
| + | <p style="font-size: 16px"> As the Lsr promoter is a useful part to many iGEM teams, we decided to add some information on a series of differential equations which can be used to model the production rate of the Lsr promoter. With this information, teams can begin to build models around AI-2 inducible systems (source here: https://doi.org/10.1371/journal.pcbi.1002172). To use these, teams must estimate a value of extracellular AI-2 concentration. For our own project we also assumed that under control of the Lsr promoter the concentration of our protein produced would be the same as the concentration of the Lsr operon proteins. We also created equations to model bacterial population and AI-2 production which can be found here: https://2021.igem.org/Team:Manchester/Model. It should be mentioned that the concentration given is an intracellular concentration so any secreted proteins must undergo further modelling or assumptions. | ||
| + | <br> | ||
| + | <br> | ||
| + | <h3 class="model-title_p2">Variables</h3> | ||
| + | |||
| + | <div style="overflow-x: auto;"> | ||
| + | <table style="background-color: #ffffff;"> | ||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th2" style="font-weight: bolder; background-color: #660099; color:#ffffff">Symbol</th> | ||
| + | <th class="model-th2" style="font-weight: bolder; background-color: #660099; color:#ffffff">Description</th> | ||
| + | <th class="model-th2" style="font-weight: bolder; background-color: #660099; color:#ffffff">Initial Value</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">t</th> | ||
| + | <th class="model-th">Time</th> | ||
| + | <th class="model-th">0 minutes</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">[OP]</th> | ||
| + | <th class="model-th">Lsr-operon concentration</th> | ||
| + | <th class="model-th">0 M</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">[REG]</th> | ||
| + | <th class="model-th">Lsr Regulator Cocnentration</th> | ||
| + | <th class="model-th">0 M</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">[R]</th> | ||
| + | <th class="model-th">LsrR Concentration</th> | ||
| + | <th class="model-th">0 M</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">[A<sub>p</sub>]</th> | ||
| + | <th class="model-th">Intracellular Phosphorylated AI-2 concentration</th> | ||
| + | <th class="model-th">0 M</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">[A<sub>out</sub>]</th> | ||
| + | <th class="model-th">Extracellular AI-2 Concentration</th> | ||
| + | <th class="model-th">Estimate/model this uM</th> | ||
| + | </tr> | ||
| + | |||
| + | </table> | ||
| + | </div> | ||
| + | <br> | ||
| + | <h3 class="model-title_p2">Parameters</h3> | ||
| + | <div style="overflow-x:auto;"> | ||
| + | <table> | ||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th" style="font-weight: bolder; background-color: #660099; color:#ffffff">Parameter</th> | ||
| + | <th class="model-th" style="font-weight: bolder; background-color: #660099; color:#ffffff">Description</th> | ||
| + | <th class="model-th" style="font-weight: bolder; background-color: #660099; color:#ffffff">Value</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">k<sub>op</sub></th> | ||
| + | <th class="model-th">Lsr-synthesis rate</th> | ||
| + | <th class="model-th">7 uM<sup>-1</sup> min<sup>-1</sup> [2]</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">k<sub>r</sub></th> | ||
| + | <th class="model-th">LsrR synthesis rate</th> | ||
| + | <th class="model-th">2 min<sup>-1</sup> [2]</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">k<sub>1</sub></th> | ||
| + | <th class="model-th">Repression Coefficient (Lsr-operon)</th> | ||
| + | <th class="model-th">0.2 uM [2]</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">k<sub>2</sub></th> | ||
| + | <th class="model-th">Repression coefficient (LsrR)</th> | ||
| + | <th class="model-th">0.1 uM [2]</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">k<sub>3</sub></th> | ||
| + | <th class="model-th">AI-2/LsrR binding rate</th> | ||
| + | <th class="model-th">0.5 uM<sup>-1</sup> min<sup>-1</sup> [2]</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">k<sub>4</sub></th> | ||
| + | <th class="model-th">Repression coefficient (Lsr-regulator)</th> | ||
| + | <th class="model-th">65 uM [2]</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">k<sub>5</sub></th> | ||
| + | <th class="model-th">REG/AI-2 interaction</th> | ||
| + | <th class="model-th">0.0001 uM<sup>-1</sup> min<sup>-1</sup> [2]</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">k<sub>f</sub></th> | ||
| + | <th class="model-th">AI-2 fluc through alternative pahways</th> | ||
| + | <th class="model-th">0.01 uM<sup>-1</sup> min<sup>-1</sup> [2]</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">k<sub>imp</sub></th> | ||
| + | <th class="model-th">AI-2 import via LasrABCD</th> | ||
| + | <th class="model-th">0.01 uM<sup>-1</sup> min<sup>-1</sup> [2]</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">nOP</th> | ||
| + | <th class="model-th">Cooperativity coefficient (Lsr-operon)</th> | ||
| + | <th class="model-th">4 [2]</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">k<sub>nR</sub></th> | ||
| + | <th class="model-th"> class="model-th"Cooperativity coefficient (LsrR)</th> | ||
| + | <th class="model-th">4 [2]</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr class="model-tr"> | ||
| + | <th class="model-th">k<sub>deg</sub></th> | ||
| + | <th class="model-th">Protein decay rate</th> | ||
| + | <th class="model-th">0.02 min<sup>-1</sup> [2]</th> | ||
| + | </tr> | ||
| + | |||
| + | </table> | ||
| + | </div> | ||
| + | <br> | ||
| + | <br> | ||
| + | <h1 class="model-title_p">Equations</h1> | ||
| + | |||
| + | <h3 class="model-title_p2">Equation 1</h3> | ||
| + | https://2021.igem.org/wiki/images/c/cc/T--Manchester--Equation_1.PNG | ||
| + | Equation 1 describes the rate of change of protein concentration produced by the Lsr promoter. Specifically this equation refers to the LsrABCD operon however in our model we assumed this concentration would be the same for our recombinant protein. | ||
| + | <br> | ||
| + | <h3 class="model-title_p2">Equation 2</h3> | ||
| + | https://2021.igem.org/wiki/images/5/5d/T--Manchester--Equation_2.PNG | ||
| + | Equation 2 is describing the rate of change of a hypothetical Lsr-regulator molecule suggested to exist in the source literature. | ||
| + | <br> | ||
| + | <h3 class="model-title_p2">Equation 3</h3> | ||
| + | https://2021.igem.org/wiki/images/7/7e/T--Manchester--Equation_3.PNG | ||
| + | Equation 3 describes the rate of synthesis of LsrR | ||
| + | <br> | ||
| + | <h3 class="model-title_p2">Equation 4</h3> | ||
| + | https://2021.igem.org/wiki/images/1/1c/T--Manchester--Equation_4.PNG | ||
| + | This equation describes the concentration of phosphorylated AI-2 inside the cell. This is contributed to by flux of AI-2 through the LsrABCD channel complex and alternative pathways. Ap is depleted by AI-2 binding to the LsrR protein and the theoretical Lsr-regulator molecule. | ||
| + | <br> | ||
| + | <h3 class="model-title_p2">Equation 5</h3> | ||
| + | https://2021.igem.org/wiki/images/2/21/T--Manchester--Equation_5.PNG | ||
| + | Equation 5 gives us the rate of decrease of the exrracellular AI-2 concentration, this assumes that we have estimated an initial concentration of AI-2 which is not very realistic. In our model we added equations to decribe bacterial concentration and AI-2 production, further details can be found here:https://2021.igem.org/Team:Manchester/Model. | ||
| + | <br> | ||
| + | <h3 class="model-title_p2">Equation 6</h3> | ||
| + | https://2021.igem.org/wiki/images/6/6d/T--Manchester--Equation_6.PNG | ||
| + | This equation gives us the rate of change of the Lsr-regulator/AI-2 complex. | ||
| + | <br> | ||
| + | <h3 class="model-title_p2">Equation 7</h3> | ||
| + | https://2021.igem.org/wiki/images/9/9e/T--Manchester--Equation_7.PNG | ||
| + | This equation gives us the rate of change of the LsrR/AI-2 complex. | ||
| + | <br> | ||
| + | |||
| + | |||
==Important notice:== | ==Important notice:== | ||
*This part is only the promoter region of LsrA gene which is normally inhibited by LsrR. It is NOT the entire LsrA gene. | *This part is only the promoter region of LsrA gene which is normally inhibited by LsrR. It is NOT the entire LsrA gene. | ||
Latest revision as of 00:48, 22 October 2021
LsrA promoter (indirectly activated by AI-2)
AI-2 quorum sensing system
The integral part of the AI-2 quorum-sensing (QS) system consists of a transporter complex, LsrABCD; its repressor, LsrR; and a cognate signal kinase, LsrK. Once uptaken into the cell, AI-2 will be phosphorylated under the effect of LsrK. Without the presence of AI-2, LsrA promoter is inhibited by the repressor, LsrR. Phosphorylated AI-2 will prevent LsrR from inhibiting this promoter, hence activating it so that the downstream sequence can be expressed. To prevent the cell from self-inducing this promoter, we must suppress its AI-2 producing capability. It can be done by knocking out LuxS, one of the vital gene required for producing AI-2. By doing that, LsrA promoter can only be activated under the presence of AI-2 from other sources than the host cell which is LuxS mutant (say, by contact with normal cells).
More information on AI-2 quorum-sensing (QS) system please refer to: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1952038
Modelling The Lsr Promoter - Contribution by Manchester iGEM 2021
As the Lsr promoter is a useful part to many iGEM teams, we decided to add some information on a series of differential equations which can be used to model the production rate of the Lsr promoter. With this information, teams can begin to build models around AI-2 inducible systems (source here: https://doi.org/10.1371/journal.pcbi.1002172). To use these, teams must estimate a value of extracellular AI-2 concentration. For our own project we also assumed that under control of the Lsr promoter the concentration of our protein produced would be the same as the concentration of the Lsr operon proteins. We also created equations to model bacterial population and AI-2 production which can be found here: https://2021.igem.org/Team:Manchester/Model. It should be mentioned that the concentration given is an intracellular concentration so any secreted proteins must undergo further modelling or assumptions.
Variables
| Symbol | Description | Initial Value |
|---|---|---|
| t | Time | 0 minutes |
| [OP] | Lsr-operon concentration | 0 M |
| [REG] | Lsr Regulator Cocnentration | 0 M |
| [R] | LsrR Concentration | 0 M |
| [Ap] | Intracellular Phosphorylated AI-2 concentration | 0 M |
| [Aout] | Extracellular AI-2 Concentration | Estimate/model this uM |
Parameters
| Parameter | Description | Value |
|---|---|---|
| kop | Lsr-synthesis rate | 7 uM-1 min-1 [2] |
| kr | LsrR synthesis rate | 2 min-1 [2] |
| k1 | Repression Coefficient (Lsr-operon) | 0.2 uM [2] |
| k2 | Repression coefficient (LsrR) | 0.1 uM [2] |
| k3 | AI-2/LsrR binding rate | 0.5 uM-1 min-1 [2] |
| k4 | Repression coefficient (Lsr-regulator) | 65 uM [2] |
| k5 | REG/AI-2 interaction | 0.0001 uM-1 min-1 [2] |
| kf | AI-2 fluc through alternative pahways | 0.01 uM-1 min-1 [2] |
| kimp | AI-2 import via LasrABCD | 0.01 uM-1 min-1 [2] |
| nOP | Cooperativity coefficient (Lsr-operon) | 4 [2] |
| knR | class="model-th"Cooperativity coefficient (LsrR) | 4 [2] |
| kdeg | Protein decay rate | 0.02 min-1 [2] |
Equations
Equation 1
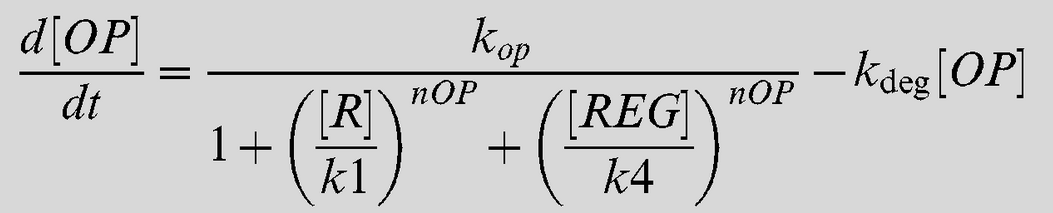
Equation 2
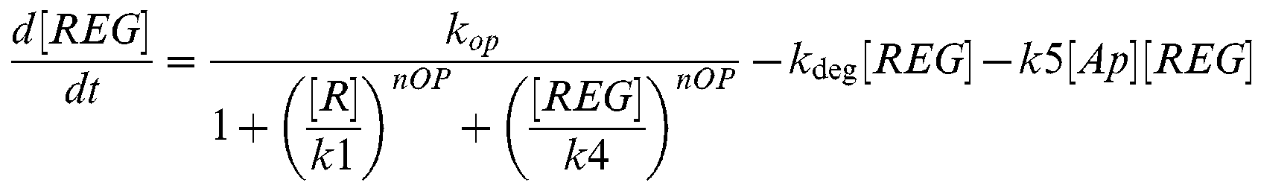
Equation 3
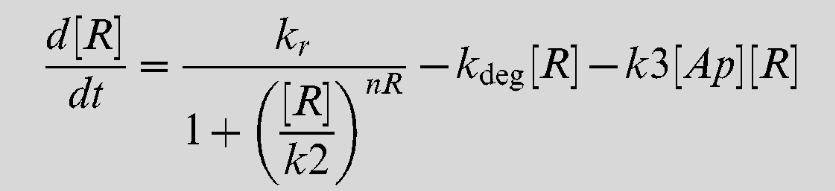
Equation 4
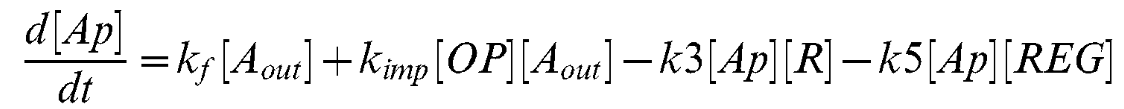
Equation 5

Equation 6
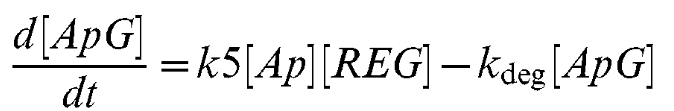
Equation 7
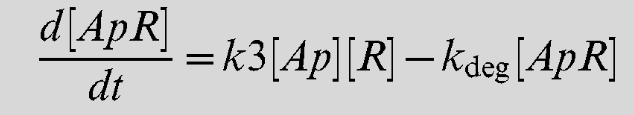
Important notice:
- This part is only the promoter region of LsrA gene which is normally inhibited by LsrR. It is NOT the entire LsrA gene.
- It is INDIRECTLY induced by AI-2, through a complex network involving LsrK,LsrR.
How this promoter works:
By ligating LsrA promoter with a protein coding sequence downstream, we can regulate the expression of that protein by AI-2 as the inducer. To prevent self-inducing by the host cell, the plasmid must be transformed into cell with LuxS gene knocked out.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]


