Difference between revisions of "Part:BBa K3113010"
Theresakeil (Talk | contribs) |
Theresakeil (Talk | contribs) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 22: | Line 22: | ||
<img src="https://2019.igem.org/wiki/images/4/4f/T--Munich--WesternBlot_CC_test.png" width="50%" class="figure-img img-fluid rounded" alt=" "> | <img src="https://2019.igem.org/wiki/images/4/4f/T--Munich--WesternBlot_CC_test.png" width="50%" class="figure-img img-fluid rounded" alt=" "> | ||
<figcaption style="font-size: 80%"> | <figcaption style="font-size: 80%"> | ||
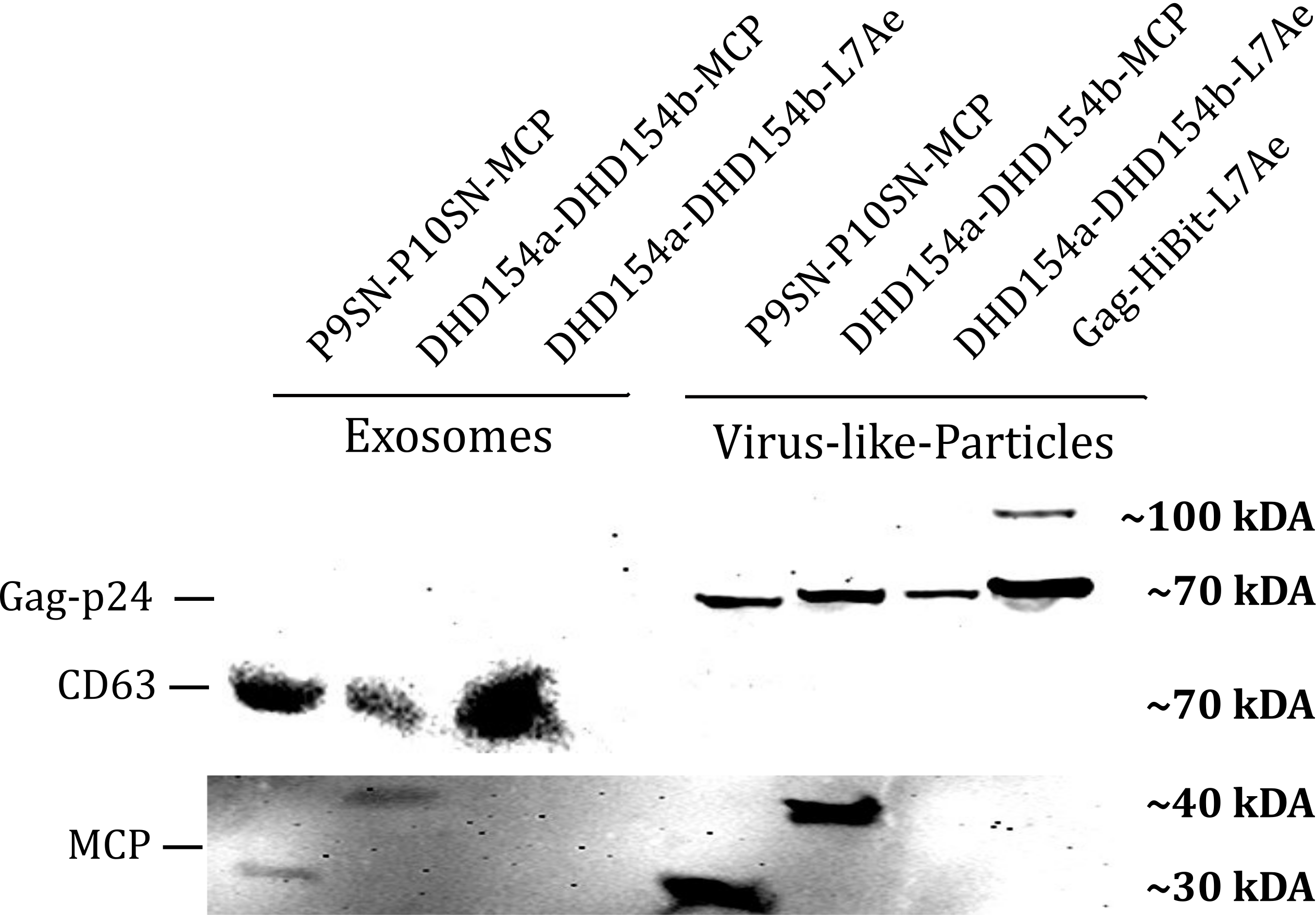
| − | <b>Figure 1: </b> | + | <b>Figure 1: </b>The presence of vesicular components modularly composed with coiled-coils and directly fused could be proven by western blotting. Top left) the exosomal marker cdc63 can be visualized with primary anti-cdc63 mouse-antibody and secondary anti-mouse antibody - horse radish peroxidase (HRP) fusion. CDC63 does not run as a tight band on the blot because of glycosylation patterns and its nature as a membrane protein. Top right) Gag-protein is determined at around 70 kDa. The fusion construct Gag-HiBiT-L7Ae shows some degradation corresponding to the molecular weight of L7Ae cleavage. Bottom) MCP RNA-binding proteins can be shown with anti-MCP antibodies. |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| Line 64: | Line 64: | ||
<figure class="figure"> | <figure class="figure"> | ||
<img src="https://2019.igem.org/wiki/images/4/4b/T--Munich--Viability_Results.png" | <img src="https://2019.igem.org/wiki/images/4/4b/T--Munich--Viability_Results.png" | ||
| − | width=" | + | width="85%" class="figure-img img-fluid rounded" alt=" "> |
<figcaption style="font-size: 80%"> | <figcaption style="font-size: 80%"> | ||
<b>Figure 5:</b> Vesicle production is not decreasing cell viability.</b> Cell viability was determined using a cytotoxicity assay at different time points after transfection to monitor possible long-term effects of VLP or exosome expression. Measurements were performed for n = 3 biological replicates. A) Cells were transfected with two different VLP constructs (Gag-CC-L7Ae, Gag-CC-MCP), a negative control to simulate transfection stress (Mock) and a positive control (untransfected). B) Cells were transfected with two different exosome constructs (CD63-L7Ae, CD63-MCP), a negative control to simulate transfection stress (Mock) and a positive control (untransfected). | <b>Figure 5:</b> Vesicle production is not decreasing cell viability.</b> Cell viability was determined using a cytotoxicity assay at different time points after transfection to monitor possible long-term effects of VLP or exosome expression. Measurements were performed for n = 3 biological replicates. A) Cells were transfected with two different VLP constructs (Gag-CC-L7Ae, Gag-CC-MCP), a negative control to simulate transfection stress (Mock) and a positive control (untransfected). B) Cells were transfected with two different exosome constructs (CD63-L7Ae, CD63-MCP), a negative control to simulate transfection stress (Mock) and a positive control (untransfected). | ||
Latest revision as of 23:54, 21 October 2019
MCP
MS2 Coat Protein (MCP) is a capsid protein that self-assembles to a capsid. The protein acts as an adapter for RNA binding into extracellular vesicles. The protein binds to the MS2-stemloop and loads mRNA into the extracellular vesicles.
Usage
The informational readout of ALiVE is RNA. To load a specific RNA into the vesicles we fused it to RNA-motifs which are bound specifically by RNA binding proteins. Two combinations of binding protein and motif were tested. One was MCP, the MS2 bacteriophage coat protein, which binds to the MS2 RNA motif.
Biology
The coat protein of the RNA bacteriophage MS2 binds a specific stem-loop structure in viral RNA to accomplish encapsidation of the genome and translational repression of replicase synthesis. [1] Bacteriophage MS2 is an icosahedral virus with 180 copies of a coat protein forming a shell around a single-stranded RNA molecule.[2]
Characterization
Western Blot
Vesicle Export in HEK293T

Expression and Export in VLPs

Expression and Export in Exosomes

Viability

Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
