Difference between revisions of "Part:BBa K3078003"
Amantadine (Talk | contribs) |
|||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 6: | Line 6: | ||
</p> | </p> | ||
</h5> | </h5> | ||
| − | |||
| − | |||
| − | |||
<h1>'''1. Usage and Biology'''</h1> | <h1>'''1. Usage and Biology'''</h1> | ||
| Line 19: | Line 16: | ||
</p> | </p> | ||
</h5> | </h5> | ||
| − | |||
<h1>'''2. Characterization'''</h1> | <h1>'''2. Characterization'''</h1> | ||
| Line 32: | Line 28: | ||
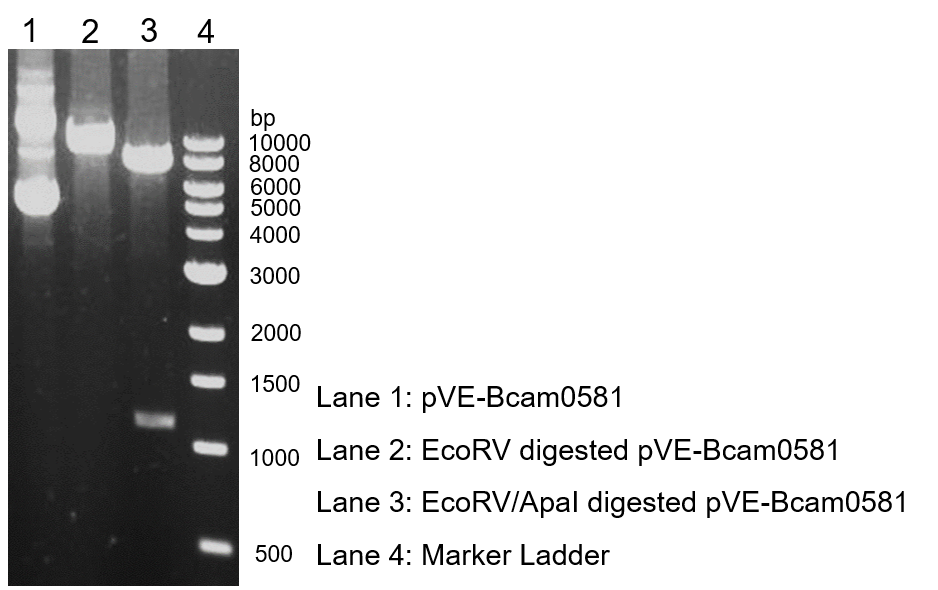
Figure 1. Digestion and electrophoresis of pVE-Bcam0581. | Figure 1. Digestion and electrophoresis of pVE-Bcam0581. | ||
</center> | </center> | ||
| − | |||
<h4>'''2.2 Expression of Bcam0581 '''</h4> | <h4>'''2.2 Expression of Bcam0581 '''</h4> | ||
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
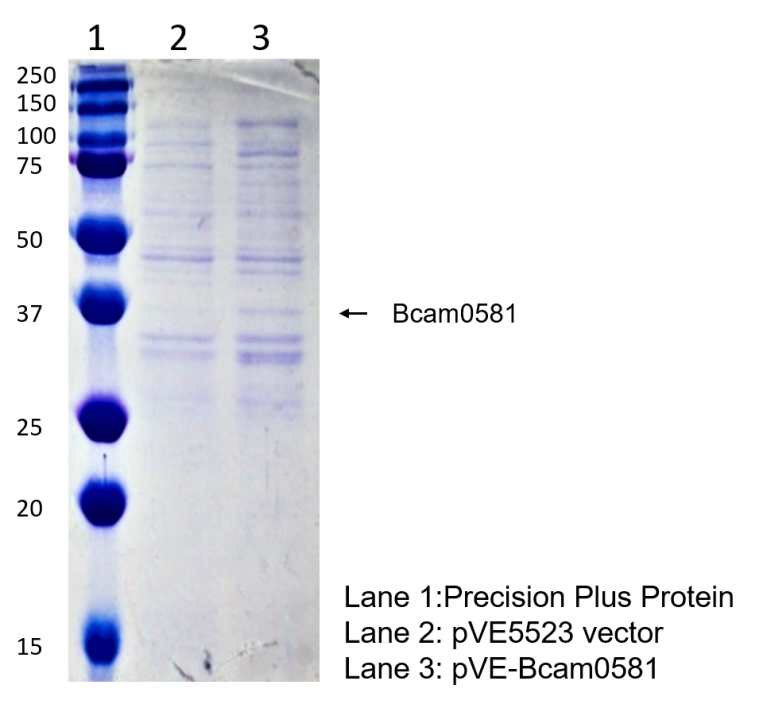
| − | To detect the expression of Bcam0581 in E. coli, the constructs were transformed into BL21(DE3). Compared to the negative control, | + | To detect the expression of Bcam0581 in <i>E. coli</i>, the constructs were transformed into BL21(DE3). Compared to the negative control, a distinct band at 37 kD was showed in pVE-Bcam0581(Figure 2). |
</p> | </p> | ||
</h5> | </h5> | ||
| Line 44: | Line 39: | ||
Figure 2. Expression of Bcam0581. The bacteria were collected and ultrasonicated. The lysate was centrifuged and supernate was electrophoresed on the SDS-PAGE gel, followed by Coomassie brilliant blue staining. | Figure 2. Expression of Bcam0581. The bacteria were collected and ultrasonicated. The lysate was centrifuged and supernate was electrophoresed on the SDS-PAGE gel, followed by Coomassie brilliant blue staining. | ||
</center> | </center> | ||
| − | |||
<h4>'''2.3 The characterized of BDSF by HPLC '''</h4> | <h4>'''2.3 The characterized of BDSF by HPLC '''</h4> | ||
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
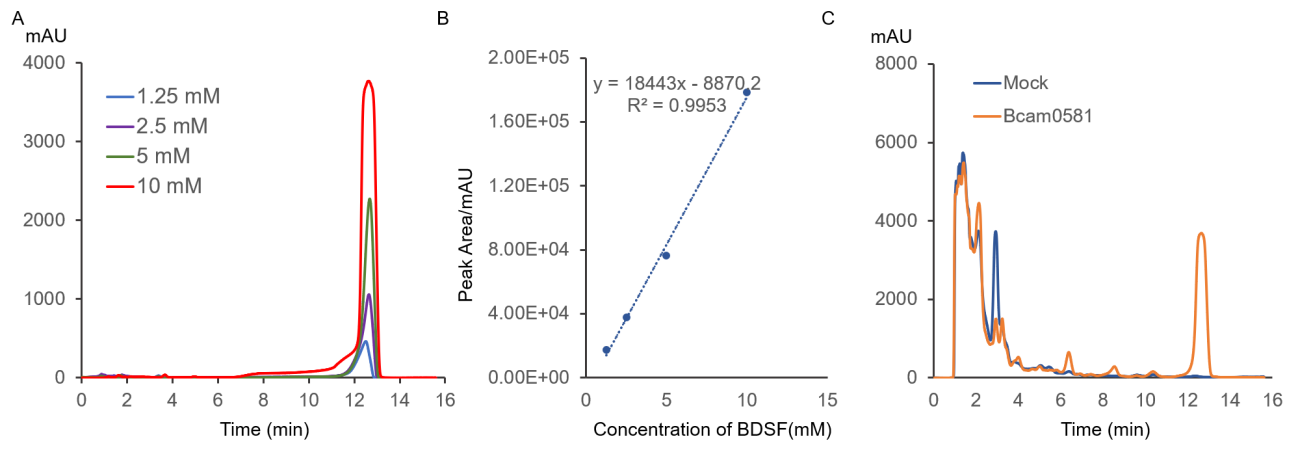
| − | + | Bcam0581 can produce the diffusible chemical BDSF that inhibits the phase transformation of <i>C. albicans</i>. The standard of BDSF concentration at 212 nm was measured with HPLC and the standard curve of BDSF concentration was established (Figure 3A&3B). To detect the concentration of BDSF which is the catalyzed production of Bcam0581, the concentrated bacteria supernatant of mock control or Bcam0581 were measured. Compared with the control , the Bcam0581 supernatant had an obvious absorption peak with a peak area of 127352 mAU·s at the retention time of 12.7 min. After calculation with the standard curve in Figure 3B, the BDSF concentration was 7.39 μM in the concentrated Bcam0581 bacteria (Figure 3C). | |
</p> | </p> | ||
</h5> | </h5> | ||
[[File:Bcam3.png|800px|center|Bcam3]] | [[File:Bcam3.png|800px|center|Bcam3]] | ||
<center style="text-align:left;"> | <center style="text-align:left;"> | ||
| − | Figure 3.A. HPLC analysis of BDSF standard with concentration of 1.25, 2.5, 5 or 10 mM | + | Figure 3.Characterization of BDSF by HPLC. A. HPLC analysis of BDSF standard with concentration of 1.25, 2.5, 5 or 10 mM. The maximum absorption peak was at 12.7 min. B. The standard curve of BDSF standard. The relationship between the peak area of BDSF standard HPLC results and BDSF in different concentrations: Peak area (mAU·s)=18443x(mM)-8870.2(mAU·s), R(sup)2(suped)=0.9953. C. HPLC of concentrated mock control or pVE-Bcam0581. The constructs were transformed into <i>E. coli</i> BL21. The bacteria were collected and ultrasonicated. The supernate from lysate was 1000 fold concentrated. |
</center> | </center> | ||
| − | + | <h4>'''2.4 Phase transformation inhibition effect of BDSF from Bcam0581 '''</h4> | |
| − | <h4>'''2.4 | + | |
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
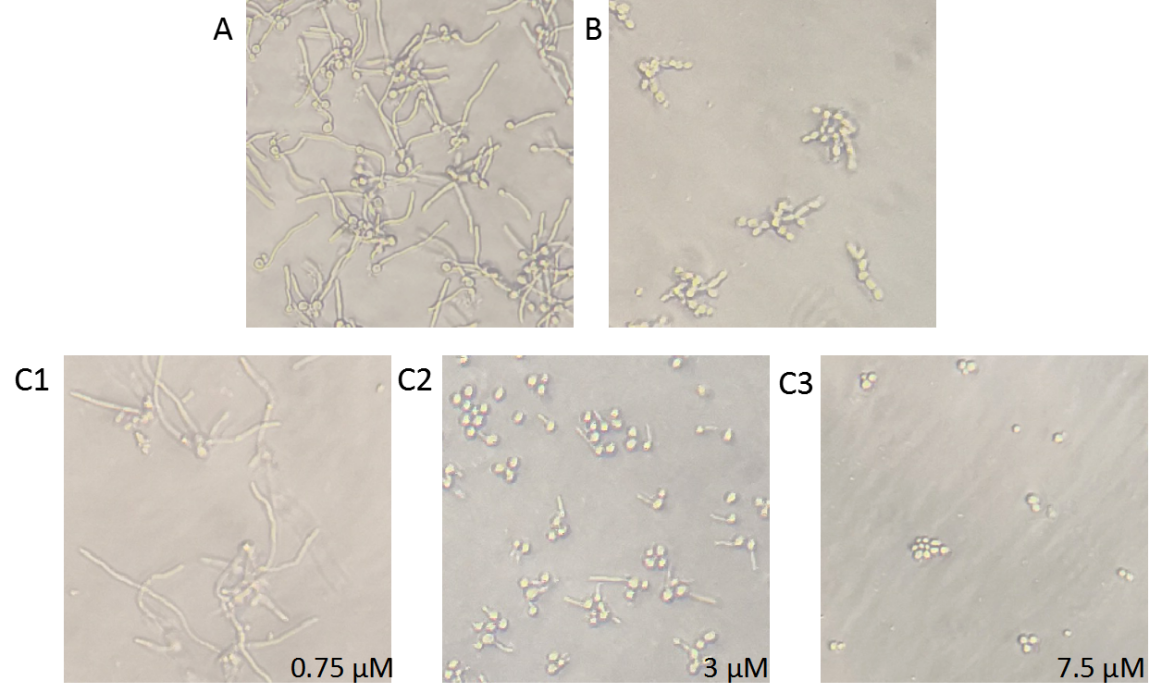
| − | + | To verify the transformation inhibition effect of BDSF, the standards or Bcam0581 bacteria supernatant were co-cultured with <i>C. albicans</i>. As shown in Figure 4. C3, 7.5 μM BDSF standards could completely inhibit the growth of hyphae. In contrast to control, the effect of the Bcam0581 bacteria supernatant’inhibition activity on phase transformation is evident.(Figure 4). | |
</p> | </p> | ||
</h5> | </h5> | ||
[[File:Bcam4.png|700px|center|Bcam4]] | [[File:Bcam4.png|700px|center|Bcam4]] | ||
<center style="text-align:left;"> | <center style="text-align:left;"> | ||
| − | Figure 4. BDSF inhibition of the phase transformation of C. albicans. C. albicans and the bacteria supernatant of control or Bcam0581 or BDSF standard were co-cultured in YPD medium supplementary with 10% serum in 96-well plate at 37℃ for 4 hours. After incubation, the hypha morphology was observed with inverted microscope | + | Figure 4. BDSF inhibition of the phase transformation of <i>C. albicans</i>. <i>C. albicans</i> and the bacteria supernatant of control or Bcam0581 or BDSF standard were co-cultured in YPD medium supplementary with 10% serum in 96-well plate at 37℃ for 4 hours. After incubation, the hypha morphology was observed with inverted microscope. A, mock control; B, bacteria supernatant of Bcam0581; C1~C3, different concentration of 0.75, 3 or 7.5 μM standard. |
</center> | </center> | ||
| − | |||
| − | |||
| − | |||
<h1>'''3. Conclusion'''</h1> | <h1>'''3. Conclusion'''</h1> | ||
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
| − | Our engineered bacteria successfully characterized Bcam0581. And it was proved that the BDSF produced by Bcam0581 was effective in inhibiting C. albicans phase transformation. | + | Our engineered bacteria successfully characterized Bcam0581. And it was proved that the BDSF produced by Bcam0581 was effective in inhibiting <i>C. albicans</i> phase transformation. |
</p> | </p> | ||
</h5> | </h5> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 102: | Line 74: | ||
<!-- --> | <!-- --> | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
| − | <partinfo> | + | <partinfo>BBa_K3078003 SequenceAndFeatures</partinfo> |
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
| − | <partinfo> | + | <partinfo>BBa_K3078003 parameters</partinfo> |
<!-- --> | <!-- --> | ||
Latest revision as of 02:21, 22 October 2019
Bcam0581
Bcam0581 protein coding region. Bcam0581 is a functional homologue of rpfF. Bcam0581 is able to produce BDSF from the acyl carrier protein (ACP) thioester of 3-hydroxydodecanoic acid, a fatty acid synthesis intermediate.
1. Usage and Biology
Bcam0581, which has the activity of dehydratase and thioesterase, can produce the diffusible chemical BDSF through the intermediate substance in the fatty acid synthesis pathway of bacteria. BDSF can inhibit the phase transformation of C. albicans.
We characterised Bcam0581 by inserting it into pVE vector.
2. Characterization
2.1 Validation of pVE-Bcam0581 construction
To verify the construction of pVE-Bcam0581 which we generated, the digestion by ApaI/EcoRV was performed by a standard protocol followed by agarose gel electrophoresis (Figure 1).
Figure 1. Digestion and electrophoresis of pVE-Bcam0581.
2.2 Expression of Bcam0581
To detect the expression of Bcam0581 in E. coli, the constructs were transformed into BL21(DE3). Compared to the negative control, a distinct band at 37 kD was showed in pVE-Bcam0581(Figure 2).
Figure 2. Expression of Bcam0581. The bacteria were collected and ultrasonicated. The lysate was centrifuged and supernate was electrophoresed on the SDS-PAGE gel, followed by Coomassie brilliant blue staining.
2.3 The characterized of BDSF by HPLC
Bcam0581 can produce the diffusible chemical BDSF that inhibits the phase transformation of C. albicans. The standard of BDSF concentration at 212 nm was measured with HPLC and the standard curve of BDSF concentration was established (Figure 3A&3B). To detect the concentration of BDSF which is the catalyzed production of Bcam0581, the concentrated bacteria supernatant of mock control or Bcam0581 were measured. Compared with the control , the Bcam0581 supernatant had an obvious absorption peak with a peak area of 127352 mAU·s at the retention time of 12.7 min. After calculation with the standard curve in Figure 3B, the BDSF concentration was 7.39 μM in the concentrated Bcam0581 bacteria (Figure 3C).
Figure 3.Characterization of BDSF by HPLC. A. HPLC analysis of BDSF standard with concentration of 1.25, 2.5, 5 or 10 mM. The maximum absorption peak was at 12.7 min. B. The standard curve of BDSF standard. The relationship between the peak area of BDSF standard HPLC results and BDSF in different concentrations: Peak area (mAU·s)=18443x(mM)-8870.2(mAU·s), R(sup)2(suped)=0.9953. C. HPLC of concentrated mock control or pVE-Bcam0581. The constructs were transformed into E. coli BL21. The bacteria were collected and ultrasonicated. The supernate from lysate was 1000 fold concentrated.
2.4 Phase transformation inhibition effect of BDSF from Bcam0581
To verify the transformation inhibition effect of BDSF, the standards or Bcam0581 bacteria supernatant were co-cultured with C. albicans. As shown in Figure 4. C3, 7.5 μM BDSF standards could completely inhibit the growth of hyphae. In contrast to control, the effect of the Bcam0581 bacteria supernatant’inhibition activity on phase transformation is evident.(Figure 4).
Figure 4. BDSF inhibition of the phase transformation of C. albicans. C. albicans and the bacteria supernatant of control or Bcam0581 or BDSF standard were co-cultured in YPD medium supplementary with 10% serum in 96-well plate at 37℃ for 4 hours. After incubation, the hypha morphology was observed with inverted microscope. A, mock control; B, bacteria supernatant of Bcam0581; C1~C3, different concentration of 0.75, 3 or 7.5 μM standard.
3. Conclusion
Our engineered bacteria successfully characterized Bcam0581. And it was proved that the BDSF produced by Bcam0581 was effective in inhibiting C. albicans phase transformation.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 338
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 26
Illegal NgoMIV site found at 43
Illegal NgoMIV site found at 284 - 1000COMPATIBLE WITH RFC[1000]