Difference between revisions of "Part:BBa K3078012"
Amantadine (Talk | contribs) |
|||
| (7 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
| − | <partinfo> | + | <partinfo>BBa_K3078012 short</partinfo> |
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
| − | + | The mutation enhanced T7 promoter. C7->A7, A9->T9. | |
</p> | </p> | ||
</h5> | </h5> | ||
| Line 10: | Line 10: | ||
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
| − | T7 promoter is one of the most common promoters. But the intensity of the wild type T7 promoter often can’t meet our demand, so we mutated it and obtained an improved T7 promoter. According to previous studies, we selected the mutation site and constructed three mutated | + | T7 promoter is one of the most common promoters. But the intensity of the wild type T7 promoter often can’t meet our demand, so we mutated it and obtained an improved T7 promoter. According to previous studies, we selected the mutation site and constructed three mutated promoters (BBa_K3078012, BBa_K3078013, BBa_K3078014)based on the wild-type T7 promoter (BBa_K2150031) into the measurement vector (BBa_K3078100). |
</p> | </p> | ||
</h5> | </h5> | ||
| − | |||
<center> | <center> | ||
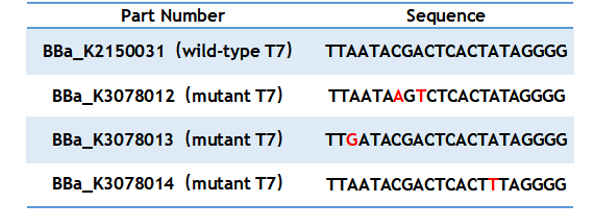
Table 1. Sequence of wild type and mutant T7 promoter. | Table 1. Sequence of wild type and mutant T7 promoter. | ||
</center> | </center> | ||
| − | [[File:T7突变-2.png|center|T7突变-2]] | + | [[File:T7-1突变.png|700px|center|T7突变-1]] |
| + | |||
| + | |||
| + | |||
| + | [[File:T7突变-2.png|700px|center|T7突变-2]] | ||
<center> | <center> | ||
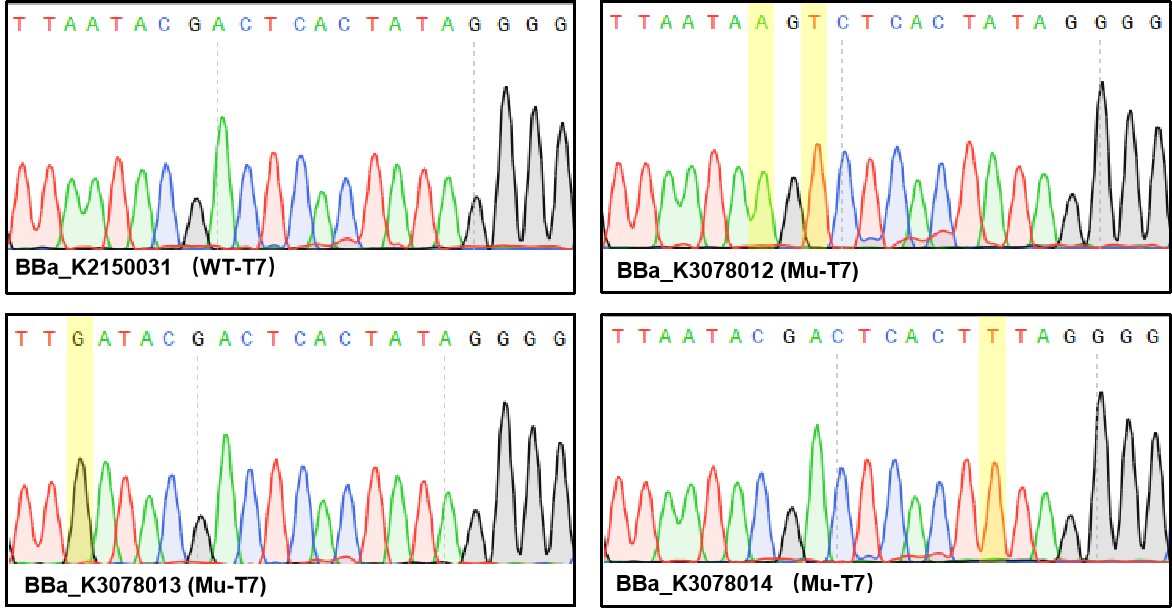
Figure 1. Sequencing the plasmids. | Figure 1. Sequencing the plasmids. | ||
| Line 26: | Line 29: | ||
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
| − | The promoter activity of wild-type and mutant T7 were measured through fluorescence intensity and normalized by OD | + | The promoter activity of wild-type and mutant T7 were measured through fluorescence intensity and normalized by OD<sub>600</sub>. BBa_K2150031 and the mutated T7 promoter lack of T7 RNA polymerase, so we added IPTG to induce the expression of T7 RNA polymerase downstream of promoter lacUV5 of on the BL21(DE3) genome, as shown in Figure 2. |
</p> | </p> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
| Line 32: | Line 35: | ||
</p> | </p> | ||
</h5> | </h5> | ||
| − | [[File:T7突变-3.png|center|T7突变-3]] | + | [[File:T7突变-3.png|700px|center|T7突变-3]] |
| − | <center> | + | <center style="text-align:left;"> |
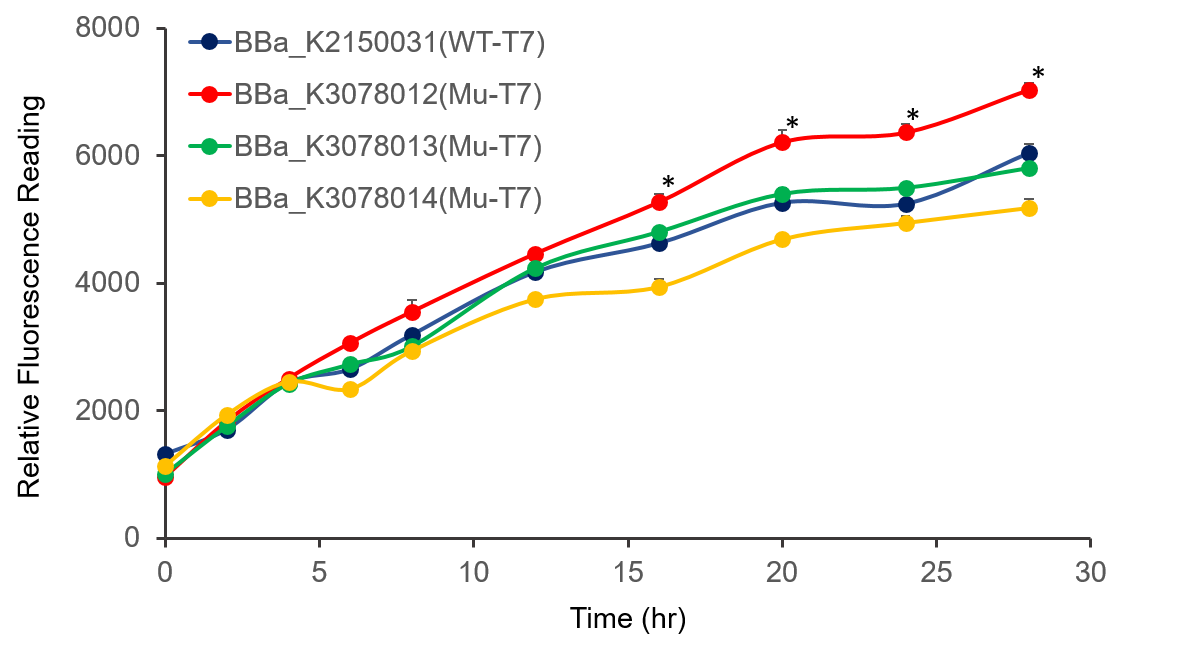
| − | Figure 2. The plasmid was transferred to | + | Figure 2. The plasmid was transferred to <i>E.coli</i> BL21, cultured overnight, and diluted OD<sub>600</sub> 0.02. IPTG was added at OD<sub>600</sub> 0.3 to make the final concentration of IPTG reach 1mM to measure at the indicated time. . |
</center> | </center> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<h1>'''3. Conclusion'''</h1> | <h1>'''3. Conclusion'''</h1> | ||
| Line 49: | Line 46: | ||
</p> | </p> | ||
</h5> | </h5> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 72: | Line 52: | ||
<!-- --> | <!-- --> | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
| − | <partinfo> | + | <partinfo>BBa_K3078012 SequenceAndFeatures</partinfo> |
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
| − | <partinfo> | + | <partinfo>BBa_K3078012 parameters</partinfo> |
<!-- --> | <!-- --> | ||
Latest revision as of 02:18, 22 October 2019
The mutation enhanced T7 promoter. C7->A7, A9->T9.
The mutation enhanced T7 promoter. C7->A7, A9->T9.
1. Design
T7 promoter is one of the most common promoters. But the intensity of the wild type T7 promoter often can’t meet our demand, so we mutated it and obtained an improved T7 promoter. According to previous studies, we selected the mutation site and constructed three mutated promoters (BBa_K3078012, BBa_K3078013, BBa_K3078014)based on the wild-type T7 promoter (BBa_K2150031) into the measurement vector (BBa_K3078100).
Table 1. Sequence of wild type and mutant T7 promoter.
Figure 1. Sequencing the plasmids.
2. Measurement
The promoter activity of wild-type and mutant T7 were measured through fluorescence intensity and normalized by OD600. BBa_K2150031 and the mutated T7 promoter lack of T7 RNA polymerase, so we added IPTG to induce the expression of T7 RNA polymerase downstream of promoter lacUV5 of on the BL21(DE3) genome, as shown in Figure 2.
It can be seen from the figure that the promoter strength of mutant is higher than wild type.
Figure 2. The plasmid was transferred to E.coli BL21, cultured overnight, and diluted OD600 0.02. IPTG was added at OD600 0.3 to make the final concentration of IPTG reach 1mM to measure at the indicated time. .
3. Conclusion
In conclusion, we constructed wild-type and mutanted T7 promoters into the measurement vector (BBa_K3078100) by Golden Gate method, and confirmed by the fluorescence measurement of the expression intensity of BBa_K3078012 was 30% higher than that of wild-type T7 promoters.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]