Difference between revisions of "Part:BBa K3078005"
Soumo sarkar (Talk | contribs) (→MIT MAHE 2020) |
|||
| (19 intermediate revisions by 4 users not shown) | |||
| Line 3: | Line 3: | ||
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
| − | + | LL-37 protein coding region. We added a stop codon to BBa_K1162006. | |
</p> | </p> | ||
| − | <h5> | + | </h5> |
| − | + | ||
| − | + | ||
| − | + | ||
<h1>'''1. Usage and Biology'''</h1> | <h1>'''1. Usage and Biology'''</h1> | ||
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
| − | + | LL-37 is an antimicrobial peptide (AMP) from the cathelicidin family of antimicrobial peptides, isolated from the Human (<i>Homo sapiens</i>). It is a 37-residue helical peptide found throughout the human body, exhibiting a broad spectrum of antimicrobial activity as well as a variety of immunomodulating effects (Dürr, Ulrich H.N., et. al 2006). It has the distinction of being the first and only cathelicidin member discovered from humans, and is extremely well studied and characterized for this reason. The peptide is produced via epithelial cells as well as leukocytes in places such as the skin, gastrointestinal tract, and the respiratory tract. This AMP is is cleaved from a larger protein, hCAP-18, in a series of modifications to yield its active form (Sorensen, O.E, et. al 2001). | |
</p> | </p> | ||
</h5> | </h5> | ||
| − | |||
<h1>'''2. Characterization'''</h1> | <h1>'''2. Characterization'''</h1> | ||
| − | <h4>'''2.1 Validation of | + | <h4>'''2.1 Validation of LL-37 construction'''</h4> |
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
| − | + | To verify the construction of pVE-LL-37 which we generated, the digestion by BglII/EcoRV was performed by a standard protocol followed by agarose gel electrophoresis (Figure 1). | |
| − | + | ||
| − | + | ||
| − | To verify the construction of pVE- | + | |
</p> | </p> | ||
</h5> | </h5> | ||
| − | [[File: | + | [[File:ll371.png|600px|center|ll37-1]] |
<center> | <center> | ||
| − | Figure 1. Digestion and | + | Figure 1. Digestion and electrophoresis of pVE-LL-37. |
</center> | </center> | ||
| − | + | <h4>'''2.2 Expression of pVE-LL-37 '''</h4> | |
| − | + | ||
| − | + | ||
| − | <h4>'''2.2 Expression of pVE- | + | |
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
| − | To | + | To detect the expression of pVE-LL-37 in <i>E. coli</i>, the constructs were transformed into BL21. Compared to the mock, there was a small size band shown in pVE-LL-37 (Figure 2). |
</p> | </p> | ||
| + | </h5> | ||
| + | [[File:ll37-2.png|600px|center|ll37-2]] | ||
| + | <center style="text-align:left;"> | ||
| + | Figure 2. Expression of pVE-LL-37. The bacteria were collected and ultrasonicated. The lysate was centrifuged and supernate was electrophoresed on the Tricine-SDS-PAGE gel, followed by Fast silver staining. The LL-37 peptide was synthesized as positive control. | ||
| + | </center> | ||
| + | |||
| + | <h4>'''2.3 Candidacidal effect of LL-37 '''</h4> | ||
| + | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
| − | + | To assess the candidacidal activity of LL-37, the spot assay was performed. As shown in Figure 13, <i>C. albicans</i> viability after LL-37 treatment was visually compared with that of control cells (no LL-37 treatment). We found that cell viability was more sensitive to LL-37. | |
</p> | </p> | ||
</h5> | </h5> | ||
| − | [[File: | + | [[File:ll37-3.png|600px|center|ll37-3]] |
| − | <center> | + | <center style="text-align:left;"> |
| − | Figure | + | Figure 3. Candidacidal activity of LL-37. <i>C. albicans</i> were collected and suspensions were resuspended to 105 cells/mL. 10 µL <i>C. albicans</i> suspension and 20 µL sterile BL21-transformed bacteria were gently mixed. After a 10-min incubation, the mixture was spotted onto the SDA agar plates. Cell viability was detected after incubation at 30 ℃ for 18 hrs. |
</center> | </center> | ||
| − | |||
| − | |||
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
| − | + | To further validate the candidacidal effect of LL-37, <i>C. albicans</i> suspensions were incubated with it. After incubation, LL-37 caused an immediate increase in PI fluorescence indicates that LL-37 can kill <i>C. albicans</i> (Figure 4). | |
</p> | </p> | ||
</h5> | </h5> | ||
| − | [[File: | + | [[File:ll37-4.png|600px|center|ll37-4]] |
| − | <center> | + | <center style="text-align:left;"> |
| − | Figure | + | Figure 4. Candidacidal activity of LL-37. 1.5 µM PI (propidiumiodide) was added into <i>C. albicans</i> suspensions (106 cells/mL, 1 mL) and serially BL21-transformed bacteria supernatant (2 mL) mixture. The whole system was incubated at 30 ℃. During the 1 h incubation, the fluorescence intensity was measured every 5 min at the excitation wavelength λexc 544 nm and emission wavelength λem 620 nm. The experiment was performed three times in triplicate. *, P < 0.05 from control using Student’s t test. |
</center> | </center> | ||
| − | |||
| − | |||
| − | |||
<h1>'''3. Conclusion'''</h1> | <h1>'''3. Conclusion'''</h1> | ||
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
| − | + | We successfully characterized LL-37 and demonstrated its ability to kill <i>C. albicans</i> from multiple angles. | |
</p> | </p> | ||
</h5> | </h5> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 95: | Line 70: | ||
<!-- --> | <!-- --> | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
| − | <partinfo> | + | <partinfo>BBa_K3078005 SequenceAndFeatures</partinfo> |
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
| − | <partinfo> | + | <partinfo>BBa_K3078005 parameters</partinfo> |
<!-- --> | <!-- --> | ||
| + | |||
| + | ==MIT_MAHE 2020== | ||
| + | '''Summary''' | ||
| + | |||
| + | LL-37 is an antimicrobial peptide (AMP) from the cathelicidin family of antimicrobial peptides. It is a 37-residue helical peptide found throughout the human body, exhibiting a broad spectrum of antimicrobial activity as well as a variety of immunomodulating effects. LL-37 is able to form aggregates in solution and lipid bilayers and thus, unlike other antimicrobial peptides, is protected from proteolytic degradation. Exposure to LL-37 results in recruitment of inflammatory cells, induction of M1 macrophages and stimulation of inflammatory responses such as inflammasome activation and type I IFN production. LL-37 has strong anti-inflammatory effects such as neutralization of TLR4 activation by LPS, downmodulation of inflammatory cytokine responses and preventing invasion and inflammatory responses to pathogenic bacteria. | ||
| + | |||
| + | ==References== | ||
| + | |||
| + | 1. Kahlenberg, J. M., & Kaplan, M. J. (2013). Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. Journal of immunology (Baltimore, Md. : 1950), 191(10), 4895–4901. https://doi.org/10.4049/jimmunol.1302005 | ||
| + | |||
| + | 2. Dürr, U. H., Sudheendra, U. S., & Ramamoorthy, A. (2006). LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochimica et biophysica acta, 1758(9), 1408–1425. https://doi.org/10.1016/j.bbamem.2006.03.030 " | ||
Latest revision as of 17:34, 23 October 2020
LL-37
LL-37 protein coding region. We added a stop codon to BBa_K1162006.
1. Usage and Biology
LL-37 is an antimicrobial peptide (AMP) from the cathelicidin family of antimicrobial peptides, isolated from the Human (Homo sapiens). It is a 37-residue helical peptide found throughout the human body, exhibiting a broad spectrum of antimicrobial activity as well as a variety of immunomodulating effects (Dürr, Ulrich H.N., et. al 2006). It has the distinction of being the first and only cathelicidin member discovered from humans, and is extremely well studied and characterized for this reason. The peptide is produced via epithelial cells as well as leukocytes in places such as the skin, gastrointestinal tract, and the respiratory tract. This AMP is is cleaved from a larger protein, hCAP-18, in a series of modifications to yield its active form (Sorensen, O.E, et. al 2001).
2. Characterization
2.1 Validation of LL-37 construction
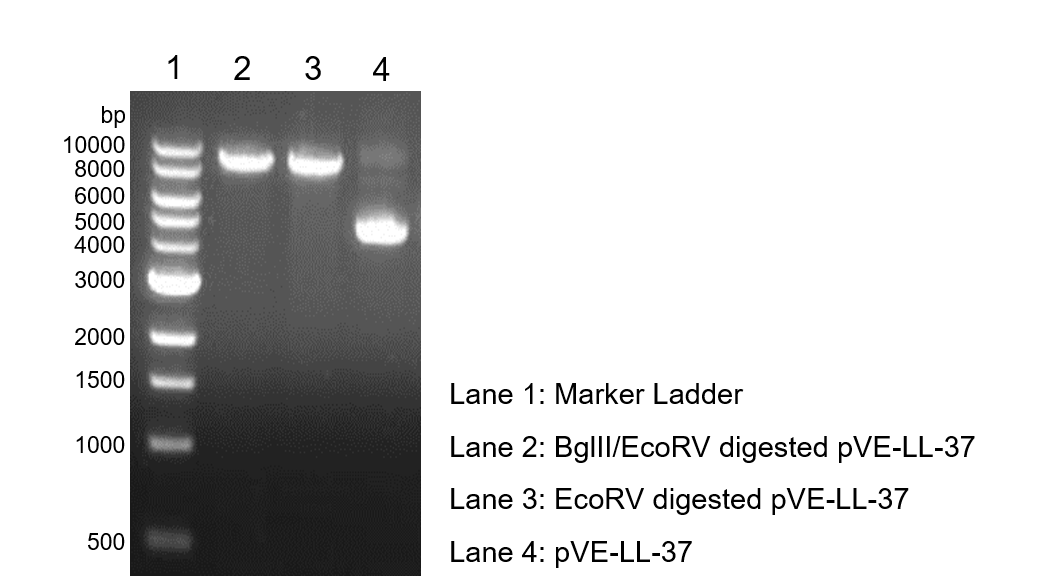
To verify the construction of pVE-LL-37 which we generated, the digestion by BglII/EcoRV was performed by a standard protocol followed by agarose gel electrophoresis (Figure 1).
Figure 1. Digestion and electrophoresis of pVE-LL-37.
2.2 Expression of pVE-LL-37
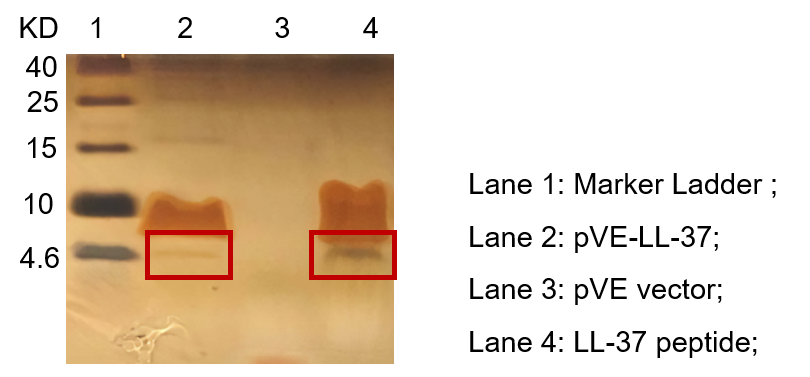
To detect the expression of pVE-LL-37 in E. coli, the constructs were transformed into BL21. Compared to the mock, there was a small size band shown in pVE-LL-37 (Figure 2).
Figure 2. Expression of pVE-LL-37. The bacteria were collected and ultrasonicated. The lysate was centrifuged and supernate was electrophoresed on the Tricine-SDS-PAGE gel, followed by Fast silver staining. The LL-37 peptide was synthesized as positive control.
2.3 Candidacidal effect of LL-37
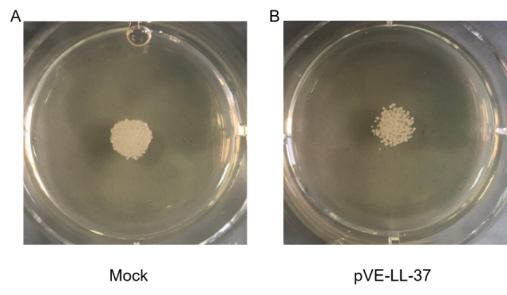
To assess the candidacidal activity of LL-37, the spot assay was performed. As shown in Figure 13, C. albicans viability after LL-37 treatment was visually compared with that of control cells (no LL-37 treatment). We found that cell viability was more sensitive to LL-37.
Figure 3. Candidacidal activity of LL-37. C. albicans were collected and suspensions were resuspended to 105 cells/mL. 10 µL C. albicans suspension and 20 µL sterile BL21-transformed bacteria were gently mixed. After a 10-min incubation, the mixture was spotted onto the SDA agar plates. Cell viability was detected after incubation at 30 ℃ for 18 hrs.
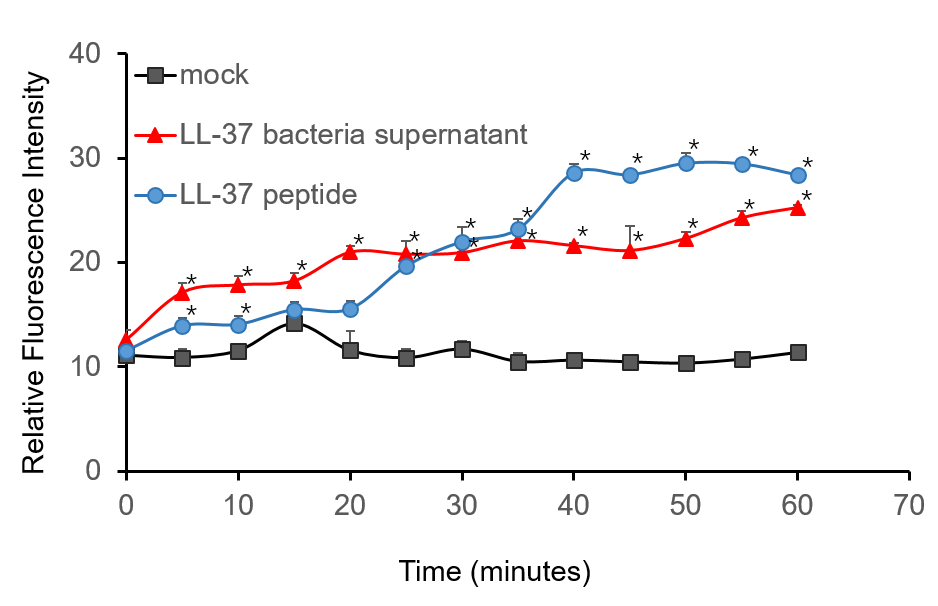
To further validate the candidacidal effect of LL-37, C. albicans suspensions were incubated with it. After incubation, LL-37 caused an immediate increase in PI fluorescence indicates that LL-37 can kill C. albicans (Figure 4).
Figure 4. Candidacidal activity of LL-37. 1.5 µM PI (propidiumiodide) was added into C. albicans suspensions (106 cells/mL, 1 mL) and serially BL21-transformed bacteria supernatant (2 mL) mixture. The whole system was incubated at 30 ℃. During the 1 h incubation, the fluorescence intensity was measured every 5 min at the excitation wavelength λexc 544 nm and emission wavelength λem 620 nm. The experiment was performed three times in triplicate. *, P < 0.05 from control using Student’s t test.
3. Conclusion
We successfully characterized LL-37 and demonstrated its ability to kill C. albicans from multiple angles.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
MIT_MAHE 2020
Summary
LL-37 is an antimicrobial peptide (AMP) from the cathelicidin family of antimicrobial peptides. It is a 37-residue helical peptide found throughout the human body, exhibiting a broad spectrum of antimicrobial activity as well as a variety of immunomodulating effects. LL-37 is able to form aggregates in solution and lipid bilayers and thus, unlike other antimicrobial peptides, is protected from proteolytic degradation. Exposure to LL-37 results in recruitment of inflammatory cells, induction of M1 macrophages and stimulation of inflammatory responses such as inflammasome activation and type I IFN production. LL-37 has strong anti-inflammatory effects such as neutralization of TLR4 activation by LPS, downmodulation of inflammatory cytokine responses and preventing invasion and inflammatory responses to pathogenic bacteria.
References
1. Kahlenberg, J. M., & Kaplan, M. J. (2013). Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. Journal of immunology (Baltimore, Md. : 1950), 191(10), 4895–4901. https://doi.org/10.4049/jimmunol.1302005
2. Dürr, U. H., Sudheendra, U. S., & Ramamoorthy, A. (2006). LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochimica et biophysica acta, 1758(9), 1408–1425. https://doi.org/10.1016/j.bbamem.2006.03.030 "