Difference between revisions of "Part:BBa K3254005"
Zongyeqing (Talk | contribs) |
|||
| (8 intermediate revisions by one other user not shown) | |||
| Line 5: | Line 5: | ||
We expected that this part can be placed between a promoter and a translational unit part and work as a normally open (NO) switch for the downstreamed gene. and switch to ON state by excising the terminator L3S3P22 between the att sites when it reacted with TG1 integrase (BBa_K2460007). Two BsaI restriction site were added between attB site and terminator. As a result, it can work as a normally closed (NC) switch for the gene which was inserted between the two BsaI site and switch to OFF state when it was excised. | We expected that this part can be placed between a promoter and a translational unit part and work as a normally open (NO) switch for the downstreamed gene. and switch to ON state by excising the terminator L3S3P22 between the att sites when it reacted with TG1 integrase (BBa_K2460007). Two BsaI restriction site were added between attB site and terminator. As a result, it can work as a normally closed (NC) switch for the gene which was inserted between the two BsaI site and switch to OFF state when it was excised. | ||
| − | + | =Usage and Biology= | |
| − | + | ==Visual Result as a Normally Open Switch== | |
| − | ==Visual Result as a Normally | + | *We conducted a simple test to see if our design met the expectation. |
| − | *We conducted a simple test to see if our design met the | + | |
===Experimental Setup=== | ===Experimental Setup=== | ||
*Genetic design principle of the experimental group is described on the page of [[Part:BBa_K3254013|BBa_K3254013]]. | *Genetic design principle of the experimental group is described on the page of [[Part:BBa_K3254013|BBa_K3254013]]. | ||
| − | *A P15A-AmpR plasmid was co- | + | *A P15A-AmpR plasmid was co-transferred into the E.coli DH5α host cell with the reporter plasmid containing this part as the negative control. |
*Single colonies were selected from the experimental LB-agar plate and negative control LB-agar plate, then inoculated into EP tubes with 500 μL M9 supplemented medium containing 500 μM IPTG for overnight growth at 37 °C and 200 rpm. | *Single colonies were selected from the experimental LB-agar plate and negative control LB-agar plate, then inoculated into EP tubes with 500 μL M9 supplemented medium containing 500 μM IPTG for overnight growth at 37 °C and 200 rpm. | ||
*Tubes were centrifuged at 10000g for 1 min. Then observed the GFP fluorescence of the cell precipitations under blue light. | *Tubes were centrifuged at 10000g for 1 min. Then observed the GFP fluorescence of the cell precipitations under blue light. | ||
| + | *M9 medium (supplemented): 6.8 g/L Na<sub>2</sub>HPO<sub>4</sub>, 3 g/L KH<sub>2</sub>PO<sub>4</sub>, 0.5 g/L NaCl, 1 g/L NH<sub>4</sub>Cl, 0.34 g/L thiamine, 0.2% casamino acids, 0.4% glucose, 2mM MgSO<sub>4</sub>, and 100 μM CaCl<sub>2</sub>. | ||
===Results=== | ===Results=== | ||
| − | *IBR-C35/F55/S37/E21/T25/G22 indicate the experimental systems for phiC31/Int5/Int7/Int8/Int10/TG1 | + | *IBR-[[Part:BBa_K3254000|C35]]/[[Part:BBa_K3254001|F55]]/[[Part:BBa_K3254002|S37]]/[[Part:BBa_K3254003|E21]]/[[Part:BBa_K3254004|T25]]/[[Part:BBa_K3254005|G22]] indicate the experimental systems for phiC31/Int5/Int7/Int8/Int10/TG1 respectively. |
*We observed the GFP fluorescence from the experimental tube as expected.<br> | *We observed the GFP fluorescence from the experimental tube as expected.<br> | ||
| − | [[File:T--GENAS_China--primary_screening.png|600px|thumb|center| ]]<br> | + | [[File:T--GENAS_China--primary_screening.png|600px|thumb|center|Visual Results as Normally Open Switches]]<br> |
| − | ==Quantitative | + | ==Quantitative Characterization of the Normally Open Switch== |
===Experimental Setup=== | ===Experimental Setup=== | ||
| Line 30: | Line 30: | ||
===Results=== | ===Results=== | ||
| − | *IBR-C35/F55/S37/E21/T25/G22 indicate the experimental systems for phiC31/Int5/Int7/Int8/Int10/TG1 | + | *IBR-C35/F55/S37/E21/T25/G22 indicate the experimental systems for phiC31/Int5/Int7/Int8/Int10/TG1 respectively. |
*Compared to other parts, this part performed well.<br> | *Compared to other parts, this part performed well.<br> | ||
| − | [[File:T--GENAS_China-- | + | [[File:T--GENAS_China--primary_quantitative_ characterization .png|600px|thumb|center|Quantitative Characterization of the Normally Open Switches]]<br> |
| + | |||
| + | ==Thermodynamic Characterization== | ||
| + | See the related information on the page of [[Part:BBa_K3254013|BBa_K3254013]]. | ||
| Line 41: | Line 44: | ||
*The composition and principle of the experimental system are indicated below. | *The composition and principle of the experimental system are indicated below. | ||
| − | [[File:T--GENAS_China--excision_with_backbone.PNG|200px|thumb|left| ]] | + | [[File:T--GENAS_China--excision_with_backbone. PNG|200px|thumb|left|The composition and principle of the experimental system]] |
===Experimental Setup=== | ===Experimental Setup=== | ||
| Line 55: | Line 58: | ||
[[File:T--GENAS_China--orthogonality_test.png]] | [[File:T--GENAS_China--orthogonality_test.png]] | ||
| − | + | ||
| + | |||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K3254005 SequenceAndFeatures</partinfo> | <partinfo>BBa_K3254005 SequenceAndFeatures</partinfo> | ||
Latest revision as of 12:42, 21 October 2019
TG1attB-BsaI site-terminator-TG1attP
We expected that this part can be placed between a promoter and a translational unit part and work as a normally open (NO) switch for the downstreamed gene. and switch to ON state by excising the terminator L3S3P22 between the att sites when it reacted with TG1 integrase (BBa_K2460007). Two BsaI restriction site were added between attB site and terminator. As a result, it can work as a normally closed (NC) switch for the gene which was inserted between the two BsaI site and switch to OFF state when it was excised.
Usage and Biology
Visual Result as a Normally Open Switch
- We conducted a simple test to see if our design met the expectation.
Experimental Setup
- Genetic design principle of the experimental group is described on the page of BBa_K3254013.
- A P15A-AmpR plasmid was co-transferred into the E.coli DH5α host cell with the reporter plasmid containing this part as the negative control.
- Single colonies were selected from the experimental LB-agar plate and negative control LB-agar plate, then inoculated into EP tubes with 500 μL M9 supplemented medium containing 500 μM IPTG for overnight growth at 37 °C and 200 rpm.
- Tubes were centrifuged at 10000g for 1 min. Then observed the GFP fluorescence of the cell precipitations under blue light.
- M9 medium (supplemented): 6.8 g/L Na2HPO4, 3 g/L KH2PO4, 0.5 g/L NaCl, 1 g/L NH4Cl, 0.34 g/L thiamine, 0.2% casamino acids, 0.4% glucose, 2mM MgSO4, and 100 μM CaCl2.
Results
- IBR-C35/F55/S37/E21/T25/G22 indicate the experimental systems for phiC31/Int5/Int7/Int8/Int10/TG1 respectively.
- We observed the GFP fluorescence from the experimental tube as expected.
Quantitative Characterization of the Normally Open Switch
Experimental Setup
- Bacteria harboring the circuits (see the top part of the result image) first inoculated from single colonies into a flat-bottom 96-well plate for overnight growth. Then, the cell cultures were diluted 1000-fold with M9 supplemented medium with 500 μM IPTG inducer and growth for another 20 hours. All incubations were carried out using a Digital Thermostatic Shaker maintained at 37 °C and 1000 rpm, using flat-bottom 96-well plates sealed with sealing film. Finally, 3-μL samples each culture were transferred to a new 96-well plate containing 200 μL per well of PBS supplemented with 2 mg/mL kanamycin.
- The fluorescence distribution of each sample was assayed using a flow cytometry. The arithmetical mean of each sample was determined using FlowJo software.
- The principle of data processing is shown on the result image.
Results
- IBR-C35/F55/S37/E21/T25/G22 indicate the experimental systems for phiC31/Int5/Int7/Int8/Int10/TG1 respectively.
- Compared to other parts, this part performed well.
Thermodynamic Characterization
See the related information on the page of BBa_K3254013.
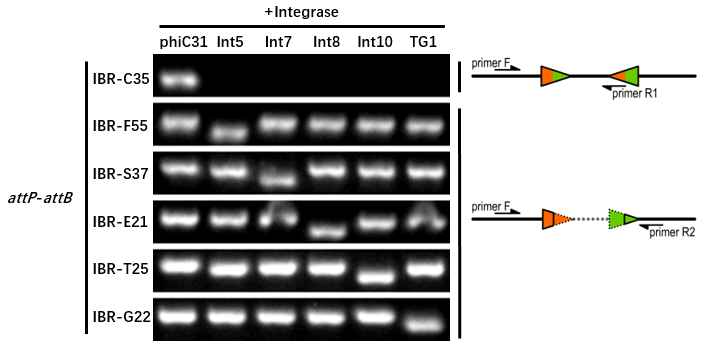
Orthogonality Characterization
Genetic Design
- The composition and principle of the experimental system are indicated below.
Experimental Setup
- The reporter plasmid contained this part were co-transferred into E.coli DH5α host with 6 integrase generator plasmids.
Then single colonies were inoculated into M9 supplemented medium for overnight growth. Then, the cell cultures were diluted 1000-fold with M9 supplemented medium with 500 μM IPTG inducer and growth for another 20 hours. All incubations were carried out using a Digital Thermostatic Shaker maintained at 37 °C and 1000 rpm, using flat-bottom 96-well plates sealed with sealing film. Finally, The cultures were sampled for genotype PCR testing.
- The principle of genotype identification was shown on the right of results image.
Results
- IBR-G22 was the plasmid containing this part.
- The result indicates that this part can only be recombined by TG1 integrase.
- The sequence (attL or attR) after recombination is GATCAGCTCCGCGGGCAAGACCGTGCTCTTACCCAGTTGGGCGGGA.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 60
Illegal BsaI.rc site found at 48