Difference between revisions of "Part:BBa K608011"
YihengYang (Talk | contribs) (→Functional Parameters: NAU-CHINA 2021) |
|||
| (45 intermediate revisions by 9 users not shown) | |||
| Line 69: | Line 69: | ||
| − | [[Image:T--SASTRA_Thanjavur-- | + | [[Image:T--SASTRA_Thanjavur--pH_final.jpg|left|500px|thumb|Effect of pH on Bba_K608011 activity]] |
Our results indicate that the fluorescence intensity was maximum at pH 7.2 (the standard pH of LB broth). Surprisingly, the intensity at pH 8 did not vary significantly from the intensity at pH 7.2 while the intensity at pH 6 deviated much more drastically. At pH values of 5 and 9, we found that the cell growth was extremely low and the absorbance at 595nm was similar to that of LB broth. However, we still proceeded to measure the fluorescence intensity at all 5 pH conditions. | Our results indicate that the fluorescence intensity was maximum at pH 7.2 (the standard pH of LB broth). Surprisingly, the intensity at pH 8 did not vary significantly from the intensity at pH 7.2 while the intensity at pH 6 deviated much more drastically. At pH values of 5 and 9, we found that the cell growth was extremely low and the absorbance at 595nm was similar to that of LB broth. However, we still proceeded to measure the fluorescence intensity at all 5 pH conditions. | ||
<br/>From our experiment, we were able to conclude that pH 7.2 and 8 were optimal conditions for the expression of our biobrick. On the other hand, pH 6 was found to be suboptimal for GFP production. We suspect that this variation in GFP intensity could be due to the effect of pH on the plasmid copy number and the binding affinity of proteins involved in the transcription and translation machineries, such as RNA polymerases and ribosomes.<br/><br/> | <br/>From our experiment, we were able to conclude that pH 7.2 and 8 were optimal conditions for the expression of our biobrick. On the other hand, pH 6 was found to be suboptimal for GFP production. We suspect that this variation in GFP intensity could be due to the effect of pH on the plasmid copy number and the binding affinity of proteins involved in the transcription and translation machineries, such as RNA polymerases and ribosomes.<br/><br/> | ||
| Line 80: | Line 80: | ||
| − | + | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
<partinfo>BBa_K608011 parameters</partinfo> | <partinfo>BBa_K608011 parameters</partinfo> | ||
| − | < | + | |
| + | |||
| + | <h1><b>Tongji_China 2019 Characterization</b></h1> | ||
| + | |||
| + | Our team tested the GFP fluorescence intensity and positive rate of PR3, PR5 and PR6 in E. coli TOP10 with a flow cytometry fluoresce (CytoFLEX LX, Beckman Coulter). | ||
| + | <br/> | ||
| + | <b>Protocol:</b> | ||
| + | <br/> | ||
| + | 1. The plasmids obtained from 5L,5M,5O,plate 1, are respectively used to transform the TOP10 strain and coated on the LB plate with chloramphenicol. The kanamycin resistant plasmid pET-28a(+) is transformed into the TOP10 strain, which is coated on the LB plate with kanamycin as a negative control. The strain is cultured overnight at 37°C. | ||
| + | <br/> | ||
| + | 2. Pick 3 colonies each plates and culture them in 5 mL LB medium with chloramphenicol 12 hours. | ||
| + | <br/> | ||
| + | 3. 4800 rmp/min centrifugate 10 minutes,using 1.5 mL PBS to dilute the sediment. | ||
| + | <br/> | ||
| + | 4. Using the negative process control sample, adjust forward-scatter and side-scatter voltages to place the strong cell peak as close to the center of the detector range as possible. | ||
| + | <br/> | ||
| + | 5. Instrument gating should be set to ensure that no cell events are discarded. | ||
| + | <br/> | ||
| + | 6. 95% negative control samples’ GFP B525-FITC-A values are less than 104. Regard those samples the GFP B525-FITC-A values more than 104 are positive. | ||
| + | <br/> | ||
| + | 7. Collect at least 5000 events per sample. | ||
| + | <br/> | ||
| + | <b>Summary:</b> | ||
| + | The results of this test show that in E. coli TOP10, PR5 has the highest GFP fluorescence intensity and positive rate among PR2, PR5 and PR6. | ||
| + | |||
| + | [[File:T--Tongji_China--BBa_K608008%2611%2612_Characterization_Fluorescence_Intensity.png|right|400px|thumb|Characterization result of BBa_K608008, BBa_K608011 and BBa_K608012, on fluorescence intensity. ]] | ||
| + | |||
| + | [[Image:T--Tongji_China--BBa_K608008%2611%2612_Characterization_Positive_Rate.png|left|thumb|400px|Characterization result of BBa_K608008, BBa_K608011 and BBa_K608012, on postive rate.]] | ||
| + | {|cellpadding="10" cellspacing="0" border="1" | ||
| + | |Sample | ||
| + | |PR2(BBa_K608008) | ||
| + | |'''PR5(BBa_K608011)''' | ||
| + | |PR6(BBa_K608012) | ||
| + | |- | ||
| + | |<span style="color:green;">GFP Fluorescence Intensity</span> | ||
| + | |24541.23 | ||
| + | |'''62923.91''' | ||
| + | |41902.30 | ||
| + | |- | ||
| + | |Fluorescence Intensity Factor | ||
| + | |1.0 | ||
| + | |'''2.6''' | ||
| + | |1.7 | ||
| + | |- | ||
| + | |<span style="color:green;">Positive Rate</span> | ||
| + | |0.7563 | ||
| + | |'''0.9190''' | ||
| + | |0.8579 | ||
| + | |} | ||
| + | |||
| + | <br/> | ||
| + | |||
| + | <h1><b>Team KCL_UK 2019</b></h1> | ||
| + | |||
| + | Our team have used this BBa_K608011 part as a fluorescent reporter to test our sRNA part. We aimed to create sRNA molecule that would bind to the GFP mRNA spanning the Ribosome Binding site and first two codons of the GFP gene. To use this BBa_K608011we first has to sub clone it into the pSB4K5 plasmid to ensure compatibility with the sRNA plasmid pSB1C3. We tested this BBa_K608011part in pSB1C3 and pSB4K5 plasmids in Xl1Blue E.coli. | ||
| + | Xl1Blue E.coli cells were transformed with these plasmids and positive colonies were selected on LB agar plates containing Kanamycin (15 ug/ml) for pSB4K5 plasmids and Chloramphenicol (34 ug/ml) for pSB1C3 plasmids respectively. Single colony from each plate was inoculated into 10 ml LB media containing appropriate antibiotic and incubated overnight in a shaking incubator at 37 oC with shaking 200 rpm. After approximately 16 h of incubation E.coli cultures were diluted 1/10 with fresh 10 ml LB media containing Kanamycin (15 ug/ml) in 20 ml universal bottle. Each experiment was performed in duplicate. At this starting point 500 ul of the culture was collected into 1.5 ml centrifuge tube, labelled 0h incubation and stored on ice. The rest of the culture was incubated for 5 h in the shaking incubator at 37 oC with shaking 200 rpm with 500 ul samples taken every hour, labelled 1, 2, 3, 4 and 5 h incubation and stored on ice. In the end 200 ul of each duplicate sample was aliquoted into black clear bottom 96 well plate. Duplicate samples of LB media containing Kanamycin and Chloramphenicol respectively were used as negative control. The OD600 and fluorescence (ex485, em520) data was recorded using PHERAstar FS (BMG Labtech) 96well plate reader. Recorded results were normalised for LB media and average results of two duplicate samples are presented in tables 1 and 1 and figures 1 and 2. | ||
| + | |||
| + | <p> | ||
| + | Table 1. OD600 of Xl1Blue E.coli cell culture harbouring pSB1C3_BBa_K608011 and pSB4K5_BBa_K608011plasmids respectively. | ||
| + | </p> | ||
| + | https://2019.igem.org/wiki/images/8/86/T--KCL_UK--characterisation_111.png | ||
| + | |||
| + | <p> | ||
| + | Table 2. GFP Fluorescence (ex485 nm, em520 nm) of Xl1Blue E.coli cell culture harbouring pSB1C3_BBa_K608011 and pSB4K5_BBa_K608011plasmids respectively. | ||
| + | </p> | ||
| + | https://2019.igem.org/wiki/images/b/b2/T--KCL_UK--characterisation_112.png | ||
| + | |||
| + | <p> | ||
| + | Figure 1. OD600 of Xl1Blue E.coli cell culture harbouring pSB1C3_BBa_K608011 and pSB4K5_BBa_K608011plasmids respectively. | ||
| + | </p> | ||
| + | https://2019.igem.org/wiki/images/8/8e/T--KCL_UK--characterisation_1_13.png | ||
| + | |||
| + | <p> | ||
| + | Figure 2. GFP Fluorescence (ex485 nm, em520 nm) of Xl1Blue E.coli cell culture harbouring pSB1C3_BBa_K608011and pSB4K5_BBa_K608011plasmids respectively. | ||
| + | </p> | ||
| + | https://2019.igem.org/wiki/images/5/59/T--KCL_UK--characterisation_1_14.png | ||
| + | |||
| + | |||
| + | <h1><b>Team University of Edinburgh 2019</b></h1> | ||
| + | '''Group: '''University of Edinburgh OG, 2019 | ||
| + | <br/> | ||
| + | '''Author: '''Francisco Ivan Rodriguez Jaubert and Cathal O’Reilly | ||
| + | <br/> | ||
| + | <br/> | ||
| + | '''Summary:''' We compared the amount of GFP expressed under the medium constitutive promoter with strong, medium and weak RBS/GFP (PR4-GFP, PR5-GFP, PR6-GFP) in TOP10, BL21 (DE3) and DH5α cells lines. We were interested in replicating the original characterisation as the Freiburg 2011 team mentioned it needed to be replicated. We did so with 3 biological replicates and 3 technical replicates for each control. Additionally, we characterised these biobricks across different common E. coli strains to investigate the consistency across these strains. | ||
| + | <br/> | ||
| + | <br/> | ||
| + | '''Methods''' | ||
| + | <br/> | ||
| + | Biobricks were extracted and amplified from the iGEM kit 2019 following standard protocols. Transformation was performed following heat-shock standard protocols. Three positive colonies were picked containing each biobrick in each different cell line. The samples were repeated in series of three to ensure consistency. This was carried out by adding 200 μl of cell culture to wells of a 96-well microplate. The plate reader was set at 485nm wavelength and 520nm emission for analysis. | ||
| + | |||
| + | |||
| + | <br/> | ||
| + | '''Results''' | ||
| + | <br/> | ||
| + | |||
| + | The next graph shows the average of the three samples (repeated three times) for each biobrick (PR4-GFP, PR5-GFP, and PR6-GFP) in different strains with their respective GFP fluorescence intensity. The weakest RBS (PR6-GFP) in BL21 presents the highest GFP absorbance, in contrast with the Freiburg data showing that the medium RBS (PR5-GFP) had the highest GFP absorbance. | ||
| + | |||
| + | |||
| + | [[Image:T--Edinburgh_OG--GFP_Flourescence_Activity_CellOrder2.png|400px|Figure 1. '''GFP Flourescence Activity (PR4-GFP, PR5-GFP, and PR6-GFP)''']] | ||
| + | |||
| + | Our data suggests that in BL21 the highest GFP expression was led by the weakest RBS (PR6-GFP), followed by the strongest RBS (PR4-GFP), and finally the medium RBS (PR5-GFP). | ||
| + | |||
| + | TOP10 shows the highest GFP expression with the strongest RBS (PR4-GFP), followed by the medium RBS (PR5-GFP), and finally the weakest RBS (PR6-GFP). | ||
| + | |||
| + | DH5a shows the highest GFP expression with the strongest RBS (PR4-GFP), followed by the weakest RBS (PR6-GFP), and finally the medium RBS (PR5-GFP). | ||
| + | |||
| + | TOP10 is the only cell line showing consistency in the hierarchy of the strongest to the weakest RBS. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ==Functional Parameters: Austin_UTexas== | ||
| + | <html> | ||
| + | <body> | ||
| + | <h3><center>Burden Imposed by this Part:</center></h3> | ||
| + | <figure> | ||
| + | <div class = "center"> | ||
| + | <center><img src = "https://static.igem.org/mediawiki/parts/4/43/T--Austin_Utexas--Low_significant_burden.png" style = "width:200px;height:120px"></center> | ||
| + | </div> | ||
| + | <figcaption><center><b>Burden Value: 13.7 ± 5.4% </b></center></figcaption> | ||
| + | </figure> | ||
| + | <p> Burden is the percent reduction in the growth rate of <i>E. coli</i> cells transformed with a plasmid containing this BioBrick (± values are 95% confidence limits). This part exhibited a significant burden. Users should be aware that BioBricks with a burden of >20-30% may be susceptible to mutating to become less functional or nonfunctional as an evolutionary consequence of this fitness cost. This risk increases as they used for more bacterial cell divisions or in larger cultures. Users should be especially careful when combining multiple burdensome parts, as plasmids with a total burden of >40% are expected to mutate so quickly that they become unclonable. Refer to any one of the | ||
| + | <a href="https://parts.igem.org/Part:BBa_K3174002">BBa_K3174002</a> - <a href="https://parts.igem.org/Part:BBa_K3174007">BBa_K3174007</a> pages for more information on the methods and other conclusions from a large-scale measurement project conducted by the <a href="https://2019.igem.org/Team:Austin_UTexas">2019 Austin_UTexas team.</a></p> | ||
| + | <p>This functional parameter was added by the <a href="https://2020.igem.org/Team:Austin_UTexas/Contribution">2020 Austin_UTexas team.</a></p> | ||
| + | </p> | ||
| + | </body> | ||
| + | </html> | ||
| + | |||
| + | |||
| + | ==Functional Parameters: NAU-CHINA 2021== | ||
| + | ===Introduction:=== | ||
| + | <p>For the contribution part of our project, we decided to do further research on the green fluorescent protein (GFP), which is commonly used as the reporter of a gene circuit. We chose the biobrick BBa_K608011 from the iGEM distribution kit, a pSB1C3 plasmid which contains a medium constitutive promoter, a medium RBS and GFP coding sequence, and transformed it into <i> E.coli</i> DH5α and <i> E.coli</i> BL21 to study the effect of temperatures and bacterial strains on the expression of GFP and the fluorescence intensity of bacteria. After that, we also extracted the GFP from the bacteria and identified the effect of pH on GFP protein activity.</p> | ||
| + | |||
| + | ===Methods:=== | ||
| + | <b>1. The effect of temperature on <i> E.coli</i> DH5α and <i> E.coli</i> BL21 containing BBa_K608011</b> | ||
| + | <p> This biobrick is presented in plasmid pSB1C3, which harbours a chloramphenicol resistant gene as a selection marker. So the Luria-Bertani (LB) plate and medium we used were supplemented with chloramphenicol (50 μg/ml). </p> | ||
| + | <p> BBa_K608011 was obtained from distribution kit and transformed into <i> E.coli</i> DH5α. Then the bacteria was coated on the LB plate, cultured overnight at 37℃. The next day we picked 4 colonies and incubated them in 5 mL LB medium for about 12 hours. The plasmids were extracted and transformed into <i> E.coli</i> BL21 and cultured overnight in 5 mL LB medium.</p> | ||
| + | <p> The overnight culture of <i>E.coli</i> BL21 and <i>E.coli</i> DH5α were diluted with fresh 150 mL LB medium and incubated at 37℃ to a 0.6 optical density at 600 nm (OD<sub>600</sub>), then separated into 3 groups, corresponding to the three temperature gradients 16, 30, 37℃. </p> | ||
| + | <p> The bacteria medium were incubated with shaking at 180 rpm and sampled every 2 hours and measured the OD<sub>600</sub> and fluorescence (ex485, em520) data using 96 well microplate reader. LB media was served as control.</p> | ||
| + | |||
| + | <b>2. The effect of pH on GFP</b> | ||
| + | <p> We took out 50mL cell culture, centrifuged at 8000rpm for 10min, and discarded the supernatant. Then, add 10mL Tris-HCl buffer to the precipitate and re-suspended. The cells were crushed by ultrasonic for 10min.The supernatant was used as GFP crude protein solution.</p> | ||
| + | <p>0.5mL crude protein solution was added to 0.5mL buffer solution of pH 3 to 11 respectively, and incubated at 37℃ for 30min. Then we measured their fluorescence intensity using microplate reader. The crude protein solution added equal ddH<sub>2</sub>O were served as control.</p> | ||
| + | |||
| + | ===Results:=== | ||
| + | <b>1. OD<sub>600</sub> and fluorescence expression levels of <i> E.coli</i> BL21 and <i> E.coli</i> DH5α at 16℃, 30℃ and 37℃</b> | ||
| + | |||
| + | <html> | ||
| + | <img src="https://2021.igem.org/wiki/images/6/6f/T--NAU-CHINA--Contribution.fig.1-2.png"width="800" height=""width="400" height=""/> | ||
| + | </html> | ||
| + | <br/> | ||
| + | Fig.1 The effect of temperature on <i> E.coli</i> DH5α and <i> E.coli</i> BL21 containing BBa_K608011. A: OD<sub>600</sub> values of bacteria at different temperatures; B: Florescence intensity of bacteria at different temperatures. | ||
| + | <br/> | ||
| + | |||
| + | <html> | ||
| + | <img src="https://2021.igem.org/wiki/images/5/57/T--NAU-CHINA--Contribution.fig.2%EF%BC%88HR%EF%BC%89.png"width="525" height=""width="262.5" height=""/> | ||
| + | </html> | ||
| + | <br/> | ||
| + | Fig.2 Florescence intensity per OD<sub>600</sub> of bacteria at different temperatures. | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <p>The data indicated that the fluorescence intensity of <i> E.coli</i> DH5α was higher than that of <i> E.coli</i> BL21 generally.</p> | ||
| + | <p> For <i> E.coli</i> DH5α, the fluorescence intensity at 16℃ was the lowest and the one at 37℃ was the highest in the first 2 hours. This is probably because cells in logarithmic growth phase, 37℃ is the optimal temperature for growth, and <i>E.coli</i> propagates fastest, so the expression of GFP was relatively high. After about 4 hours, the fluorescence intensity at 16℃is gradually higher than those at 30℃ and 37℃, that is because 16℃ is the optimal temperature for protein expression. </p> | ||
| + | <p> For BL21, the fluorescence intensity was highest at 37℃ comparatively. </p> | ||
| + | <p> The results can be used for reference in the study of GFP expression of these two stains of <i> E.coli</i>. Before the OD<sub>600</sub> value of DH5α culture is up to 0.8, it can be cultured at 37℃ first to multiply, and then decrease to 16℃ to increase GFP expression. In this way, the total amount of GFP expression in <i> E.coli </i> DH5α medium can reach the optimal level. But for the BL21 stain, the temperature for cells’ growth can be maintained at 37℃. 16℃ and 30℃ are the most commonly used industrial fermentation temperatures, and 37℃ is the most commonly used temperature for bacterial culture. Characterization of BBa_K608011 at these three temperatures can be used as a guide for industrial and laboratory applications. </p> | ||
| + | <br/> | ||
| + | <b>2. Fluorescence expression levels of GFP under different pH conditions. </b> | ||
| + | |||
| + | <html> | ||
| + | <img src="https://2021.igem.org/wiki/images/0/01/T--NAU-CHINA--Contribution.fig.3%283%29.png"width="525" height=""width="262.5" height=""/> | ||
| + | </html> | ||
| + | <br/> | ||
| + | Fig.3 Florescence intensity of GFP at different pH. | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <p> The fluorescence intensity of blank control was similar to the one at pH 8. Surprisingly, the intensity of specimens at pH higher than 7 did not vary significantly from the intensity of which at pH 7, even higher, while the ones at lower pH deviated much more drastically. </p> | ||
| + | <p> Our experiment indicated that GFP could acquire higher fluorescence intensity at neutral or alkaline conditions. On the contrary, since acid has negative influence on GFP fluorescence intensity, we should pay attention to control the pH of cell culture medium higher than 7. Bacteria have a variety of secretion systems, and fluorescent proteins can also confirm the secretion efficiency of signal peptides. Once secreted, the protein is in an in vitro environment, so it is very important to characterize the nature of GFP in vitro environment. Our results suggested that GFP’s conformation can keep stable in alkaline conditions, which means it could be used in studying the functions of some proteins or polypeptides under extreme conditions. Our characterization is also useful for cell-free systems. On the contrary, since acid has negative influence on GFP fluorescence intensity, we should pay attention to control the pH of cell culture medium higher than 7. </p> | ||
Latest revision as of 16:29, 21 October 2021
Medium promoter with medium RBS and GFP
Medium promoter from the constitutive promoter family combined with medium RBS (PR5) and GFP. To quantify the gene expression, GFP was tagged to the promoter RBS domain.
The GFP fluorescence was measured with a plate reader:
The fluorescence intensity and protein concentration were measured with the FLUOstar Omega,
which is a multi-mode microplate reader.
Samples were pipetted into the microplate and analyzed via the plate reader. In this experiment we focused on the protein concentration and the fluorescence intensity of RFP.
We measured the protein concentration with the bradford-assay. This is a method to determine the total protein
concentration. To analyze the protein concentration of the samples, Coomassie Brillant Blue was pippeted to each sample. With the binding of the dye to the proteins the color changes from dark red to blue. The more protein in the solution the more Coomassie dye can bind to proteins and the more the color changes into blue. The absorption of bound Coomassie dye is 595nm. The absorbance is proportional with the amount of bound dye. With a series of Bovine Serum Albumin (BSA) measurements the exact protein concentration of the samples can be determined. BSA acts like a “marker” because the concentration of BSA is known and with a linear calibration line the exact protein concentration can be detected.
GFP served as a reporter of expression. We wanted to know how strong the promoter and RBS activity is. With this reporter gene it was possible to analyze the expression via plate reader. GFP is excited at a wavelength of 509nm and has an emission of 520nm. The plate reader illuminates the samples with a high energy xenon flash lamp. Optical filters or monochromator create the exact wavelength. The more GFP in the sample the higher is the GFP fluorescence intensity. The intensity is collected with the second optical system and is detected with a side window photomultiplier tube.
Promoter and RBS:
PR1: strong Promoter (J23104) strong RBS (B0034)
PR2: strong Promoter (J23104) medium RBS (B0032)
PR3: strong Promoter (J23104) weak RBS (B0031)
PR4: medium Promoter (J23110) strong RBS (B0034)
PR5: medium Promoter (J23110) medium RBS (B0032)
PR6: medium Promoter (J23110) weak RBS (B0031)
| sample | PR2 | PR3 | PR4 | PR5 | PR6 |
| GFP fluorescence intensity | 11378.5 | 1445.0 | 4596.2 | 41221.1 | 26922.7 |
| factor | 7.9 | 1.0 | 3.2 | 28.5 | 18.6 |
The results of this test show that PR5 has 28.5 times higher expression of GFP in comparison with with PR3 which has the lowest expression. The fluorescence intensity of GFP varies, and because of lack of time we could not repeat this experiment. We have also tested the promotor and RBS activity with RFP as a reporter and the results deviate from this experiment. So we are looking forward to test this system another time.
SASTRA_Thanjavur 2019 Characterization - Effect of pH on promoter strength
In our experiment, we decided to study the effect of pH on biobrick BBa_K608011. This biobrick consists of a medium constitutive promoter (Bba_J23110) and a medium RBS (Bba_B0032) along with a reporter Green Fluorescent Protein (Bba_E0040).
We began our experiment with the transformation of the biobrick into E. coli DH5alpha cells. We then ventured into identifying the effect of pH on the biobrick’s activity. We know that the expression of the reporter protein relies on the plasmid copy number, binding affinity of ribosomes and RNA polymerase. These mechanisms depend on various environmental conditions including pH. We hypothesized that the expression of GFP would be maximum at an optimal pH of 7.2 and would subsequently decrease as the pH deviated from the optimal one, thus producing a bell-shaped curve when the pH is plotted against the GFP intensity.
This biobrick is present in plasmid pSB1C3, which harbours a chloramphenicol resistant gene for the selection of our transformants. Following the selection of our transformants, we decided to optimize the antibiotic concentration to achieve a balance between cell growth and plasmid selection.
We found out that the chloramphenicol concentration of 20ug/mL was the optimal concentration for our transformants and decided to keep this as our standard for all further experiments.
To characterize the biobrick, we decided to test its activity under different pH conditions. To achieve the optimal absorbance, our transformants were grown for 48 hours due to their slow growth rate, which can be attributed to the metabolic burden the plasmid imposes on the cells. The fluorescence was measured using a spectrofluorometer with excitation and emission wavelengths of 485nm and 520 nm respectively. This step was done to detect transformants that expressed GFP. Our experiment was performed in triplicates with LB media as our control to measure the background fluorescence.
Our results indicate that the fluorescence intensity was maximum at pH 7.2 (the standard pH of LB broth). Surprisingly, the intensity at pH 8 did not vary significantly from the intensity at pH 7.2 while the intensity at pH 6 deviated much more drastically. At pH values of 5 and 9, we found that the cell growth was extremely low and the absorbance at 595nm was similar to that of LB broth. However, we still proceeded to measure the fluorescence intensity at all 5 pH conditions.
From our experiment, we were able to conclude that pH 7.2 and 8 were optimal conditions for the expression of our biobrick. On the other hand, pH 6 was found to be suboptimal for GFP production. We suspect that this variation in GFP intensity could be due to the effect of pH on the plasmid copy number and the binding affinity of proteins involved in the transcription and translation machineries, such as RNA polymerases and ribosomes.
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 706
Functional Parameters
Tongji_China 2019 Characterization
Our team tested the GFP fluorescence intensity and positive rate of PR3, PR5 and PR6 in E. coli TOP10 with a flow cytometry fluoresce (CytoFLEX LX, Beckman Coulter).
Protocol:
1. The plasmids obtained from 5L,5M,5O,plate 1, are respectively used to transform the TOP10 strain and coated on the LB plate with chloramphenicol. The kanamycin resistant plasmid pET-28a(+) is transformed into the TOP10 strain, which is coated on the LB plate with kanamycin as a negative control. The strain is cultured overnight at 37°C.
2. Pick 3 colonies each plates and culture them in 5 mL LB medium with chloramphenicol 12 hours.
3. 4800 rmp/min centrifugate 10 minutes,using 1.5 mL PBS to dilute the sediment.
4. Using the negative process control sample, adjust forward-scatter and side-scatter voltages to place the strong cell peak as close to the center of the detector range as possible.
5. Instrument gating should be set to ensure that no cell events are discarded.
6. 95% negative control samples’ GFP B525-FITC-A values are less than 104. Regard those samples the GFP B525-FITC-A values more than 104 are positive.
7. Collect at least 5000 events per sample.
Summary:
The results of this test show that in E. coli TOP10, PR5 has the highest GFP fluorescence intensity and positive rate among PR2, PR5 and PR6.
| Sample | PR2(BBa_K608008) | PR5(BBa_K608011) | PR6(BBa_K608012) |
| GFP Fluorescence Intensity | 24541.23 | 62923.91 | 41902.30 |
| Fluorescence Intensity Factor | 1.0 | 2.6 | 1.7 |
| Positive Rate | 0.7563 | 0.9190 | 0.8579 |
Team KCL_UK 2019
Our team have used this BBa_K608011 part as a fluorescent reporter to test our sRNA part. We aimed to create sRNA molecule that would bind to the GFP mRNA spanning the Ribosome Binding site and first two codons of the GFP gene. To use this BBa_K608011we first has to sub clone it into the pSB4K5 plasmid to ensure compatibility with the sRNA plasmid pSB1C3. We tested this BBa_K608011part in pSB1C3 and pSB4K5 plasmids in Xl1Blue E.coli. Xl1Blue E.coli cells were transformed with these plasmids and positive colonies were selected on LB agar plates containing Kanamycin (15 ug/ml) for pSB4K5 plasmids and Chloramphenicol (34 ug/ml) for pSB1C3 plasmids respectively. Single colony from each plate was inoculated into 10 ml LB media containing appropriate antibiotic and incubated overnight in a shaking incubator at 37 oC with shaking 200 rpm. After approximately 16 h of incubation E.coli cultures were diluted 1/10 with fresh 10 ml LB media containing Kanamycin (15 ug/ml) in 20 ml universal bottle. Each experiment was performed in duplicate. At this starting point 500 ul of the culture was collected into 1.5 ml centrifuge tube, labelled 0h incubation and stored on ice. The rest of the culture was incubated for 5 h in the shaking incubator at 37 oC with shaking 200 rpm with 500 ul samples taken every hour, labelled 1, 2, 3, 4 and 5 h incubation and stored on ice. In the end 200 ul of each duplicate sample was aliquoted into black clear bottom 96 well plate. Duplicate samples of LB media containing Kanamycin and Chloramphenicol respectively were used as negative control. The OD600 and fluorescence (ex485, em520) data was recorded using PHERAstar FS (BMG Labtech) 96well plate reader. Recorded results were normalised for LB media and average results of two duplicate samples are presented in tables 1 and 1 and figures 1 and 2.
Table 1. OD600 of Xl1Blue E.coli cell culture harbouring pSB1C3_BBa_K608011 and pSB4K5_BBa_K608011plasmids respectively.
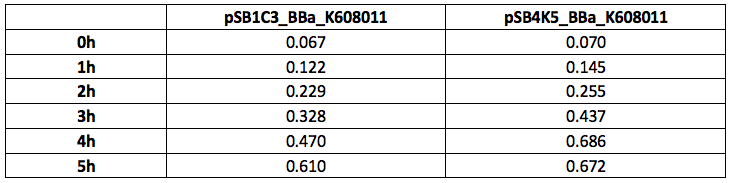
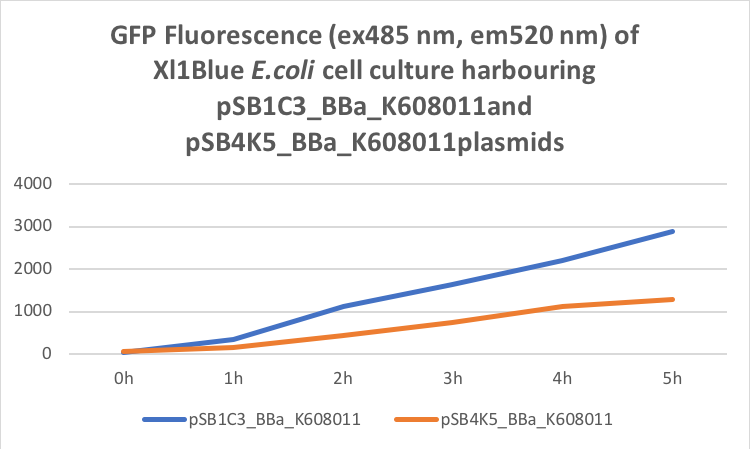
Table 2. GFP Fluorescence (ex485 nm, em520 nm) of Xl1Blue E.coli cell culture harbouring pSB1C3_BBa_K608011 and pSB4K5_BBa_K608011plasmids respectively.

Figure 1. OD600 of Xl1Blue E.coli cell culture harbouring pSB1C3_BBa_K608011 and pSB4K5_BBa_K608011plasmids respectively.
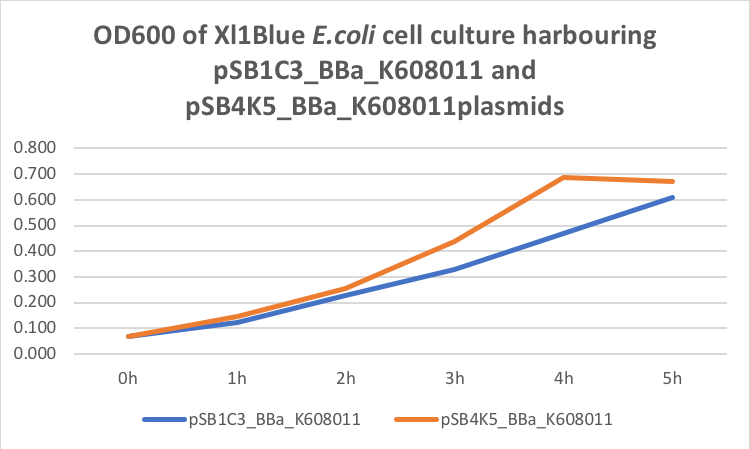
Figure 2. GFP Fluorescence (ex485 nm, em520 nm) of Xl1Blue E.coli cell culture harbouring pSB1C3_BBa_K608011and pSB4K5_BBa_K608011plasmids respectively.
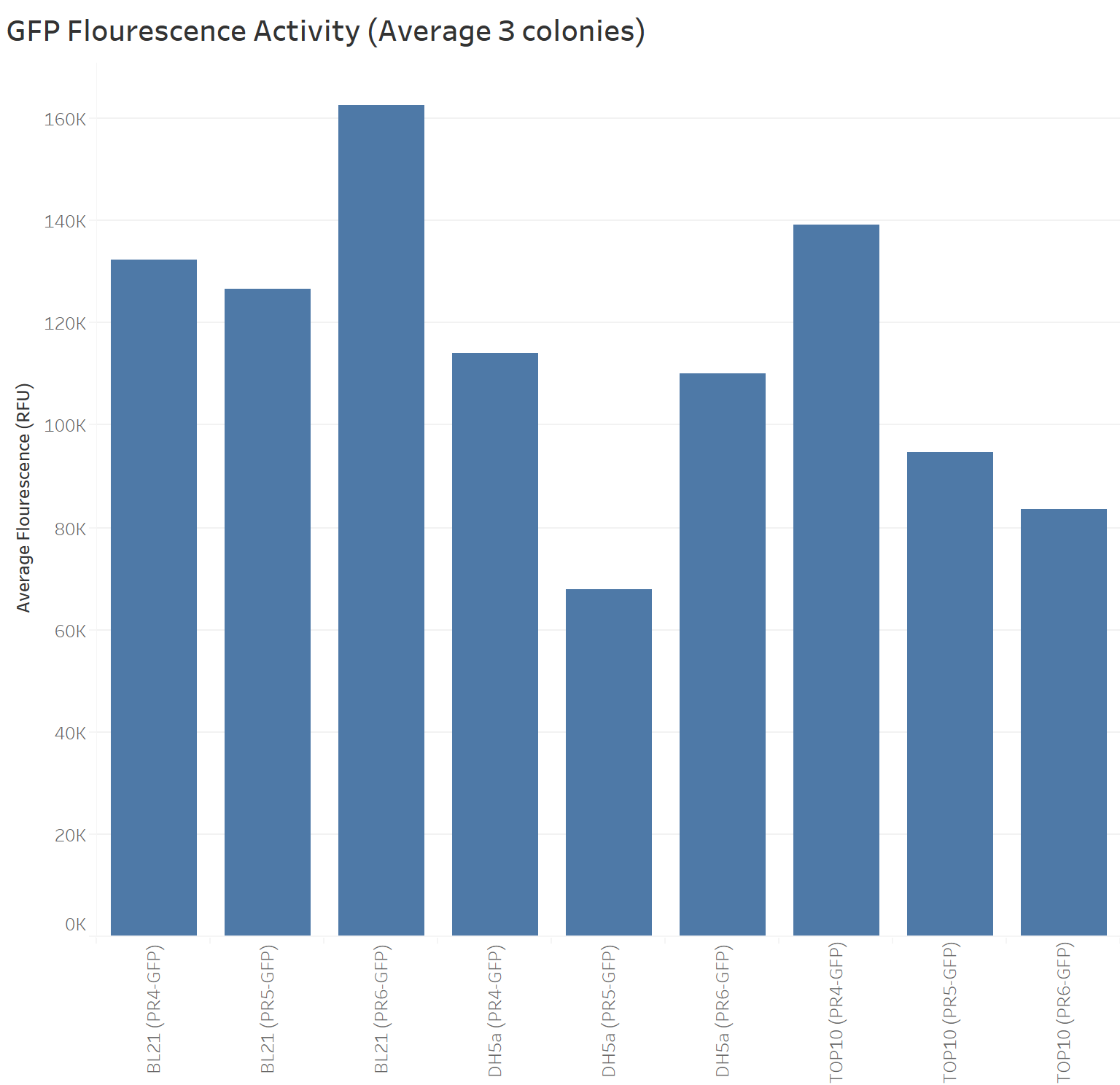
Team University of Edinburgh 2019
Group: University of Edinburgh OG, 2019
Author: Francisco Ivan Rodriguez Jaubert and Cathal O’Reilly
Summary: We compared the amount of GFP expressed under the medium constitutive promoter with strong, medium and weak RBS/GFP (PR4-GFP, PR5-GFP, PR6-GFP) in TOP10, BL21 (DE3) and DH5α cells lines. We were interested in replicating the original characterisation as the Freiburg 2011 team mentioned it needed to be replicated. We did so with 3 biological replicates and 3 technical replicates for each control. Additionally, we characterised these biobricks across different common E. coli strains to investigate the consistency across these strains.
Methods
Biobricks were extracted and amplified from the iGEM kit 2019 following standard protocols. Transformation was performed following heat-shock standard protocols. Three positive colonies were picked containing each biobrick in each different cell line. The samples were repeated in series of three to ensure consistency. This was carried out by adding 200 μl of cell culture to wells of a 96-well microplate. The plate reader was set at 485nm wavelength and 520nm emission for analysis.
Results
The next graph shows the average of the three samples (repeated three times) for each biobrick (PR4-GFP, PR5-GFP, and PR6-GFP) in different strains with their respective GFP fluorescence intensity. The weakest RBS (PR6-GFP) in BL21 presents the highest GFP absorbance, in contrast with the Freiburg data showing that the medium RBS (PR5-GFP) had the highest GFP absorbance.
Our data suggests that in BL21 the highest GFP expression was led by the weakest RBS (PR6-GFP), followed by the strongest RBS (PR4-GFP), and finally the medium RBS (PR5-GFP).
TOP10 shows the highest GFP expression with the strongest RBS (PR4-GFP), followed by the medium RBS (PR5-GFP), and finally the weakest RBS (PR6-GFP).
DH5a shows the highest GFP expression with the strongest RBS (PR4-GFP), followed by the weakest RBS (PR6-GFP), and finally the medium RBS (PR5-GFP).
TOP10 is the only cell line showing consistency in the hierarchy of the strongest to the weakest RBS.
Functional Parameters: Austin_UTexas
Burden Imposed by this Part:

Burden is the percent reduction in the growth rate of E. coli cells transformed with a plasmid containing this BioBrick (± values are 95% confidence limits). This part exhibited a significant burden. Users should be aware that BioBricks with a burden of >20-30% may be susceptible to mutating to become less functional or nonfunctional as an evolutionary consequence of this fitness cost. This risk increases as they used for more bacterial cell divisions or in larger cultures. Users should be especially careful when combining multiple burdensome parts, as plasmids with a total burden of >40% are expected to mutate so quickly that they become unclonable. Refer to any one of the BBa_K3174002 - BBa_K3174007 pages for more information on the methods and other conclusions from a large-scale measurement project conducted by the 2019 Austin_UTexas team.
This functional parameter was added by the 2020 Austin_UTexas team.
Functional Parameters: NAU-CHINA 2021
Introduction:
For the contribution part of our project, we decided to do further research on the green fluorescent protein (GFP), which is commonly used as the reporter of a gene circuit. We chose the biobrick BBa_K608011 from the iGEM distribution kit, a pSB1C3 plasmid which contains a medium constitutive promoter, a medium RBS and GFP coding sequence, and transformed it into E.coli DH5α and E.coli BL21 to study the effect of temperatures and bacterial strains on the expression of GFP and the fluorescence intensity of bacteria. After that, we also extracted the GFP from the bacteria and identified the effect of pH on GFP protein activity.
Methods:
1. The effect of temperature on E.coli DH5α and E.coli BL21 containing BBa_K608011
This biobrick is presented in plasmid pSB1C3, which harbours a chloramphenicol resistant gene as a selection marker. So the Luria-Bertani (LB) plate and medium we used were supplemented with chloramphenicol (50 μg/ml).
BBa_K608011 was obtained from distribution kit and transformed into E.coli DH5α. Then the bacteria was coated on the LB plate, cultured overnight at 37℃. The next day we picked 4 colonies and incubated them in 5 mL LB medium for about 12 hours. The plasmids were extracted and transformed into E.coli BL21 and cultured overnight in 5 mL LB medium.
The overnight culture of E.coli BL21 and E.coli DH5α were diluted with fresh 150 mL LB medium and incubated at 37℃ to a 0.6 optical density at 600 nm (OD600), then separated into 3 groups, corresponding to the three temperature gradients 16, 30, 37℃.
The bacteria medium were incubated with shaking at 180 rpm and sampled every 2 hours and measured the OD600 and fluorescence (ex485, em520) data using 96 well microplate reader. LB media was served as control.
2. The effect of pH on GFP
We took out 50mL cell culture, centrifuged at 8000rpm for 10min, and discarded the supernatant. Then, add 10mL Tris-HCl buffer to the precipitate and re-suspended. The cells were crushed by ultrasonic for 10min.The supernatant was used as GFP crude protein solution.
0.5mL crude protein solution was added to 0.5mL buffer solution of pH 3 to 11 respectively, and incubated at 37℃ for 30min. Then we measured their fluorescence intensity using microplate reader. The crude protein solution added equal ddH2O were served as control.
Results:
1. OD600 and fluorescence expression levels of E.coli BL21 and E.coli DH5α at 16℃, 30℃ and 37℃

Fig.1 The effect of temperature on E.coli DH5α and E.coli BL21 containing BBa_K608011. A: OD600 values of bacteria at different temperatures; B: Florescence intensity of bacteria at different temperatures.

Fig.2 Florescence intensity per OD600 of bacteria at different temperatures.
The data indicated that the fluorescence intensity of E.coli DH5α was higher than that of E.coli BL21 generally.
For E.coli DH5α, the fluorescence intensity at 16℃ was the lowest and the one at 37℃ was the highest in the first 2 hours. This is probably because cells in logarithmic growth phase, 37℃ is the optimal temperature for growth, and E.coli propagates fastest, so the expression of GFP was relatively high. After about 4 hours, the fluorescence intensity at 16℃is gradually higher than those at 30℃ and 37℃, that is because 16℃ is the optimal temperature for protein expression.
For BL21, the fluorescence intensity was highest at 37℃ comparatively.
The results can be used for reference in the study of GFP expression of these two stains of E.coli. Before the OD600 value of DH5α culture is up to 0.8, it can be cultured at 37℃ first to multiply, and then decrease to 16℃ to increase GFP expression. In this way, the total amount of GFP expression in E.coli DH5α medium can reach the optimal level. But for the BL21 stain, the temperature for cells’ growth can be maintained at 37℃. 16℃ and 30℃ are the most commonly used industrial fermentation temperatures, and 37℃ is the most commonly used temperature for bacterial culture. Characterization of BBa_K608011 at these three temperatures can be used as a guide for industrial and laboratory applications.
2. Fluorescence expression levels of GFP under different pH conditions.

Fig.3 Florescence intensity of GFP at different pH.
The fluorescence intensity of blank control was similar to the one at pH 8. Surprisingly, the intensity of specimens at pH higher than 7 did not vary significantly from the intensity of which at pH 7, even higher, while the ones at lower pH deviated much more drastically.
Our experiment indicated that GFP could acquire higher fluorescence intensity at neutral or alkaline conditions. On the contrary, since acid has negative influence on GFP fluorescence intensity, we should pay attention to control the pH of cell culture medium higher than 7. Bacteria have a variety of secretion systems, and fluorescent proteins can also confirm the secretion efficiency of signal peptides. Once secreted, the protein is in an in vitro environment, so it is very important to characterize the nature of GFP in vitro environment. Our results suggested that GFP’s conformation can keep stable in alkaline conditions, which means it could be used in studying the functions of some proteins or polypeptides under extreme conditions. Our characterization is also useful for cell-free systems. On the contrary, since acid has negative influence on GFP fluorescence intensity, we should pay attention to control the pH of cell culture medium higher than 7.