Difference between revisions of "Part:BBa K3168006"
CMichielsen (Talk | contribs) |
EvaHanckmann (Talk | contribs) |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 10: | Line 10: | ||
===Characterization=== | ===Characterization=== | ||
====Expression==== | ====Expression==== | ||
| − | Cysteine-free-NanoLuc was cloned into a pET28a(+) vector, subsequently expressed in BL21 (DE3) E. coli and purified using Ni-NTA affinity chromatography and Strep-Tactin purification. Afterwards, expression was analyzed on SDS-PAGE ( | + | Cysteine-free-NanoLuc was cloned into a pET28a(+) vector, subsequently expressed in BL21 (DE3) E. coli and purified using Ni-NTA affinity chromatography and Strep-Tactin purification. Afterwards, expression was analyzed on SDS-PAGE (Figure 1). This shows that after the purification there is a clear blob between 20 kDa and 25 kDa which corresponds with the molecular weight of the 6xHis-tagged and Strep-tagged Cysteine-free-NanoLuc, which is 22 kDa. |
[[File:T--TU_Eindhoven--SDSCysFreeNL.png|50px|]] | [[File:T--TU_Eindhoven--SDSCysFreeNL.png|50px|]] | ||
| Line 17: | Line 17: | ||
====Functionality test==== | ====Functionality test==== | ||
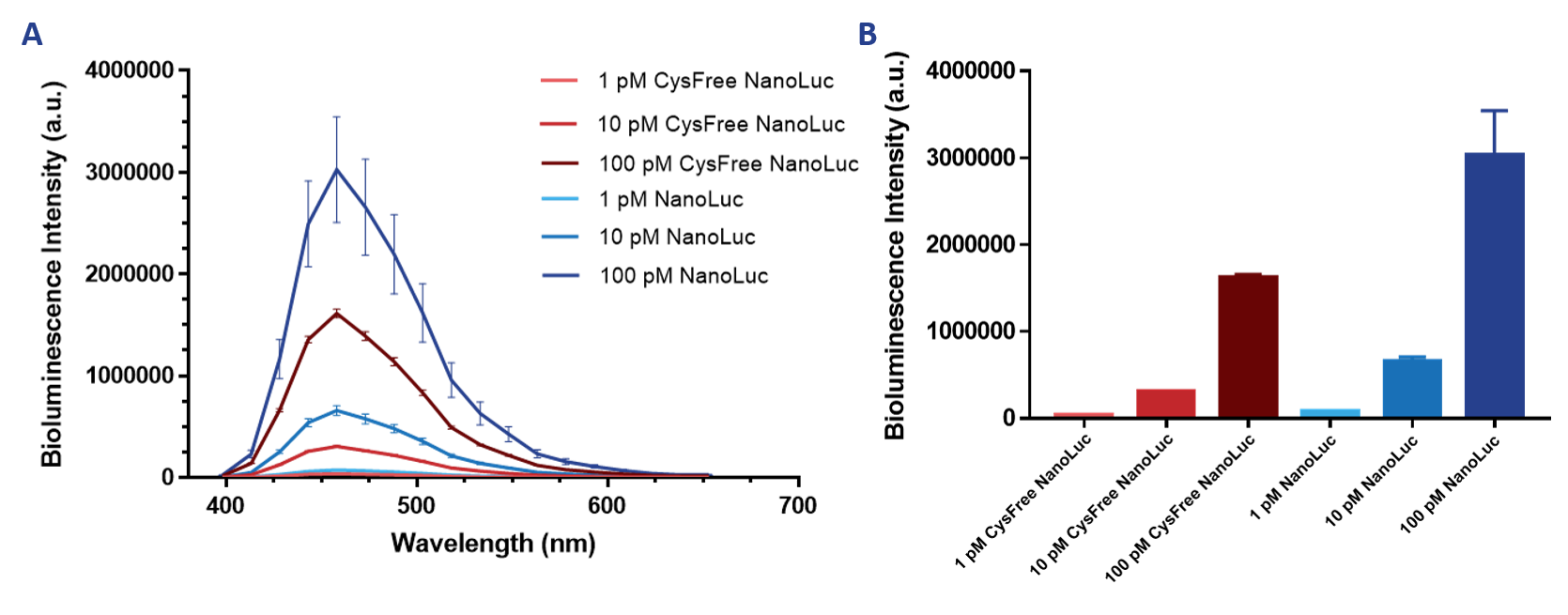
| − | After successful expression and purification, measurements could be performed to determine whether cysteine-free-NanoLuc was still functional and could be used as a replacement for NanoLuc in fusion proteins where subsequent maleimide coupling is required. Bioluminescence intensity was measured for different concentrations of both NanoLuc proteins (Figure 2). This shows that cysteine-free-NanoLuc has a similar shape of the emission spectrum and has its maximum at the same wavelength as NanoLuc. | + | After successful expression and purification, measurements could be performed to determine whether cysteine-free-NanoLuc was still functional and could be used as a replacement for NanoLuc in fusion proteins where subsequent maleimide coupling is required. Bioluminescence intensity was measured for different concentrations of both NanoLuc proteins (Figure 2). This shows that cysteine-free-NanoLuc has a similar shape of the emission spectrum and has its maximum at the same wavelength as NanoLuc. Furthermore, it shows that cysteine-free-NanoLuc is still functional, although a decrease of about fifty percent in intensity for all measured concentrations can be observed. However, due to NanoLuc’s initial brightness, cysteine-free-NanoLuc is still much brighter than most other luciferases while also maintaining its small size and high stability. |
| − | [[File:T--TU_Eindhoven--NLvsCysFreeNL.png| | + | [[File:T--TU_Eindhoven--NLvsCysFreeNL.png|800px|]] |
| − | ''Figure 2. Emission spectrum ( | + | ''Figure 2. Emission spectrum (A) and maximum intensity at 460 nm (B) of Cysteine-free-NanoLuc and NanoLuc.'' |
===References=== | ===References=== | ||
Latest revision as of 15:13, 21 October 2019
Cysteine-free-NanoLuc
NanoLuc is a deep-sea shrimp-derived luciferase, which is smaller compared to the Firefly and Renilla luciferases and therefore offers certain advantages over the traditional methods. NanoLuc has increased stability, smaller size and a >150-fold increase in luminescence (England, 2016). Furthermore, NanoLuc displays high physical stability, maintains its activity during incubation up to 55 oC or in culture medium for >15 h at 37 oC and shows no evidence of posttranslational modifications or subcellular partitioning in mammalian cells (Hall, 2012). Furimazine, NanoLuc’s substrate, shows increased stability and lower background activity which enhances the possibilities for bioluminescence imaging (England, 2016). Furimazine reacts with NanoLuc in the presence of oxygen. Furimazine is converted to Furimamide and a blue luminescence output occurs. Furthermore, a flexible (SGG)2 linker is located in front of NanoLuc to enable the formation of fusion proteins. A strep-tag is also included at the end for protein purification.
NanoLuc originally contains one cysteine, which was mutated to a serine to create a cysteine-free construct. Hereby, this NanoLuc can be used in the formation of fusion proteins which can be labeled via maleimide coupling. This is useful for BRET (Bioluminescence Resonance Energy Transfer) based sensors.
Usage and Biology
NanoLuc has potential to play a great role in disease detection, molecular imaging, and therapeutic monitoring (England, 2016). NanoLuc is used a lot in the development of BRET-sensors (Arts, 2017).
Characterization
Expression
Cysteine-free-NanoLuc was cloned into a pET28a(+) vector, subsequently expressed in BL21 (DE3) E. coli and purified using Ni-NTA affinity chromatography and Strep-Tactin purification. Afterwards, expression was analyzed on SDS-PAGE (Figure 1). This shows that after the purification there is a clear blob between 20 kDa and 25 kDa which corresponds with the molecular weight of the 6xHis-tagged and Strep-tagged Cysteine-free-NanoLuc, which is 22 kDa.
Figure 1. SDS-PAGE of Cysteine-free-NanoLuc (319 µM) after purification.
Functionality test
After successful expression and purification, measurements could be performed to determine whether cysteine-free-NanoLuc was still functional and could be used as a replacement for NanoLuc in fusion proteins where subsequent maleimide coupling is required. Bioluminescence intensity was measured for different concentrations of both NanoLuc proteins (Figure 2). This shows that cysteine-free-NanoLuc has a similar shape of the emission spectrum and has its maximum at the same wavelength as NanoLuc. Furthermore, it shows that cysteine-free-NanoLuc is still functional, although a decrease of about fifty percent in intensity for all measured concentrations can be observed. However, due to NanoLuc’s initial brightness, cysteine-free-NanoLuc is still much brighter than most other luciferases while also maintaining its small size and high stability.
Figure 2. Emission spectrum (A) and maximum intensity at 460 nm (B) of Cysteine-free-NanoLuc and NanoLuc.
References
Arts, R., Aper, S. J., & Merkx, M. (2017). Engineering BRET-sensor proteins. In Methods in enzymology (Vol. 589, pp. 87-114). Academic Press.
England, C. G., Ehlerding, E. B., & Cai, W. (2016). NanoLuc: a small luciferase is brightening up the field of bioluminescence. Bioconjugate chemistry, 27(5), 1175-1187.
Hall, M. P., Unch, J., Binkowski, B. F., Valley, M. P., Butler, B. L., Wood, M. G., ... & Robers, M. B. (2012). Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS chemical biology, 7(11), 1848-1857.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]