Difference between revisions of "Part:BBa K3128001"
(→Sequence and features) |
(→Construction) |
||
| (19 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
==Sequence and features== | ==Sequence and features== | ||
<div style="text-align:justify;"> | <div style="text-align:justify;"> | ||
| − | A reporter | + | A reporter biobrick was constructed to be used with a BATCH assays. This reporter plasmid is composed of a cAMP dependant promoter (CAP dependant) driving the expression of a reporter gene.<br> |
| − | + | A very well characterized iGEM part was used : [https://parts.igem.org/Part:BBa_J04450 BBa_J04450].<br> | |
| − | Characterization of this BioBrick done by | + | Characterization of this BioBrick done by [https://2019.igem.org/Team:Grenoble-Alpes/Contribution Team Grenoble-Alpes 2019]showed that the '''lactose promoter''' present in this BioBrick, which makes this BioBrick useful for our system. |
===Construction=== | ===Construction=== | ||
| − | + | BglII and BamHI restriction restriction sites were added on both 5’ and 3’ ends of the Red Fluorescent Protein gene as follow: | |
| Line 14: | Line 14: | ||
| − | The mRFP1 was removed and replaced by the | + | The mRFP1 was removed and replaced by the NanoLuciferase gene previously amplified from a commercial vector. |
| − | Nano Luciferase was chosen for its ability to produce luminescence | + | Nano Luciferase was chosen for its ability to produce luminescence in absence of ATP (ATP being the substrate of Adenylate Cyclase (AC)), it must be as available as possible and so not be used by our reporter protein) and for the high level of luminescence observed for a single protein . |
</div> | </div> | ||
| Line 22: | Line 22: | ||
==Usage and Biology== | ==Usage and Biology== | ||
| − | |||
===Bacterial Adenylate Cyclase Two-Hybrid (BACTH)=== | ===Bacterial Adenylate Cyclase Two-Hybrid (BACTH)=== | ||
<div style="text-align:justify;"> | <div style="text-align:justify;"> | ||
| Line 31: | Line 30: | ||
<br>This system involves the <i>Bordetella pertussis</i> '''adenylate cyclase''' which is the responsible agent for the pertussis disease. | <br>This system involves the <i>Bordetella pertussis</i> '''adenylate cyclase''' which is the responsible agent for the pertussis disease. | ||
| − | Adenylate cyclase catalytic domain has the particularity to be splittable in two distinct parts: '''T18''' and '''T25''' sub-parts, <u>unable to | + | Adenylate cyclase catalytic domain has the particularity to be splittable in two distinct parts: '''T18''' and '''T25''' sub-parts, <u>unable to function unless they |
reassociate.</u> Each sub-part of the enzyme is fused with a protein of interest, either the bait or the prey protein chose beforehand by the experimentator. <br> | reassociate.</u> Each sub-part of the enzyme is fused with a protein of interest, either the bait or the prey protein chose beforehand by the experimentator. <br> | ||
| Line 38: | Line 37: | ||
</span> | </span> | ||
| − | <br> | + | <br>When two proteins interact, then '''T18''' and '''T25''' are bring together and reconstitute a <u>functional adenylate cyclase enzyme</u> thus enabling cAMP production. Using |
<i>cya-</i> bacteria<i> – strain for whom the adenylate gene is deleted, involving an absence of this endogenous enzyme – </i> a BACTH could be done with the creation of two | <i>cya-</i> bacteria<i> – strain for whom the adenylate gene is deleted, involving an absence of this endogenous enzyme – </i> a BACTH could be done with the creation of two | ||
fusion proteins : the first one, fused at its N or C terminal intracellular end with the '''T18 sub-part'''; the second one fused with the '''T25 sub-part'''. <br> | fusion proteins : the first one, fused at its N or C terminal intracellular end with the '''T18 sub-part'''; the second one fused with the '''T25 sub-part'''. <br> | ||
| Line 49: | Line 48: | ||
<div style="text-align:justify;"> | <div style="text-align:justify;"> | ||
| − | + | '''[https://2019.igem.org/Team:Grenoble-Alpes NeuroDrop]''' detection system is based on the use of a '''BACTH'''. The point is to allow the induction of the gene only when the two sub-parts of AC are physically close, which only occurs when the target is present in the sample. The reassembly of AC enables cAMP production, followed by the '''transcription of the reporter''' that is under a '''CAP-cAMP dependent promoter'''. <br> | |
| + | To use this system, an '''AC deficient bacteria strain''' (BTH101) that is not able to produce endogenous cAMP is needed to prevent any transcription from CAP dependant promoter in abscence of interaction between both sub-parts. <br> | ||
| + | For the promoter, we decided to use the '''lactose promoter''' (a CAP dependent promoter), we have demonstrated its repression in the absence of an exogenous source of cAMP (in the AC deficient bacterial strain). | ||
| Line 57: | Line 58: | ||
==References== | ==References== | ||
<i> | <i> | ||
| − | |||
| − | |||
| − | |||
Fields S, Song O. A novel genetic system to detect protein–protein interactions. Nature. (1989)<br> | Fields S, Song O. A novel genetic system to detect protein–protein interactions. Nature. (1989)<br> | ||
Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. PNAS. (1998)<br> | Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. PNAS. (1998)<br> | ||
Karimova G, Gauliard E, Davi M, P.Ouellette S, Ladant D. Protein–Protein Interaction: Bacterial Two-Hybrid. (2017)<br> | Karimova G, Gauliard E, Davi M, P.Ouellette S, Ladant D. Protein–Protein Interaction: Bacterial Two-Hybrid. (2017)<br> | ||
| − | Picture of the reaction ATP-cAMP. Khan Academy Website. Retrieved October 10, 2019, from https://www.khanacademy.org | + | Picture of the reaction ATP-cAMP. Khan Academy Website. Retrieved October 10, 2019, from https://www.khanacademy.org<br> |
| − | Euromedex, BACTH System Kit | + | Euromedex, BACTH System Kit <br> |
Leusch, Paulaitis, Friedman. Adenylate cyclase toxin of Bordetella pertussis: production, purification, and partial characterization. Am Soc Microbiol | Infect Immun. (1990) <br> | Leusch, Paulaitis, Friedman. Adenylate cyclase toxin of Bordetella pertussis: production, purification, and partial characterization. Am Soc Microbiol | Infect Immun. (1990) <br> | ||
Hantke, Winkler, Schultz. Escherichia coli exports cyclic AMP via TolC. J Bacteriol. (2011) <br> | Hantke, Winkler, Schultz. Escherichia coli exports cyclic AMP via TolC. J Bacteriol. (2011) <br> | ||
| − | |||
</i> | </i> | ||
Latest revision as of 11:52, 15 October 2019
NanoLuciferase reporter for BACTH assay
Contents
Sequence and features
A reporter biobrick was constructed to be used with a BATCH assays. This reporter plasmid is composed of a cAMP dependant promoter (CAP dependant) driving the expression of a reporter gene.
A very well characterized iGEM part was used : BBa_J04450.
Characterization of this BioBrick done by Team Grenoble-Alpes 2019showed that the lactose promoter present in this BioBrick, which makes this BioBrick useful for our system.
Construction
BglII and BamHI restriction restriction sites were added on both 5’ and 3’ ends of the Red Fluorescent Protein gene as follow:

The mRFP1 was removed and replaced by the NanoLuciferase gene previously amplified from a commercial vector.
Nano Luciferase was chosen for its ability to produce luminescence in absence of ATP (ATP being the substrate of Adenylate Cyclase (AC)), it must be as available as possible and so not be used by our reporter protein) and for the high level of luminescence observed for a single protein .
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
Bacterial Adenylate Cyclase Two-Hybrid (BACTH)
The principle lies on the interaction-mediated reconstitution of a signalling cascade in Escherichia coli. The messenger molecule involved in this cascade is the
cyclic adenosine monophosphate (cAMP) produced by the adenylate cyclase. Adenylate cyclase is an enzyme catalysing the cAMP production from ATP. It
physiologically participates to the cellular transmission.
This system involves the Bordetella pertussis adenylate cyclase which is the responsible agent for the pertussis disease.
Adenylate cyclase catalytic domain has the particularity to be splittable in two distinct parts: T18 and T25 sub-parts, unable to function unless they
reassociate. Each sub-part of the enzyme is fused with a protein of interest, either the bait or the prey protein chose beforehand by the experimentator.
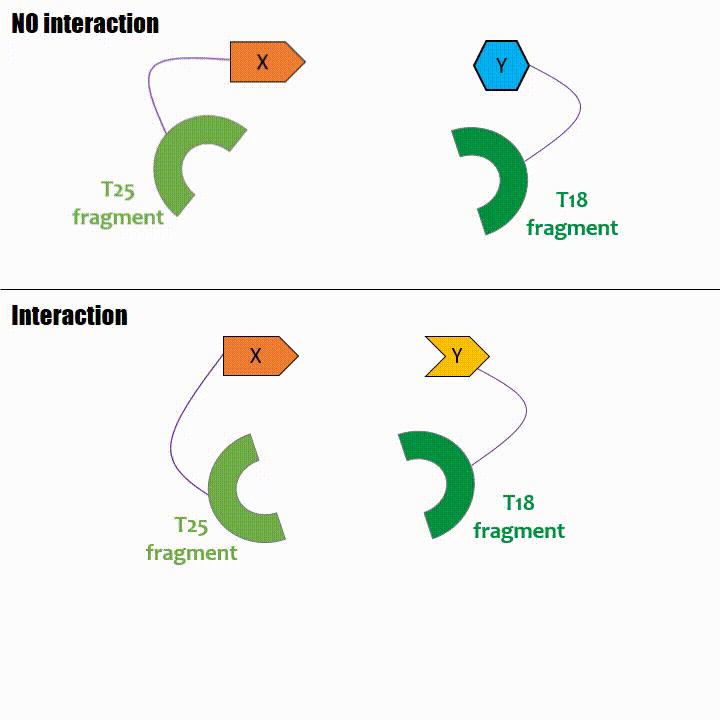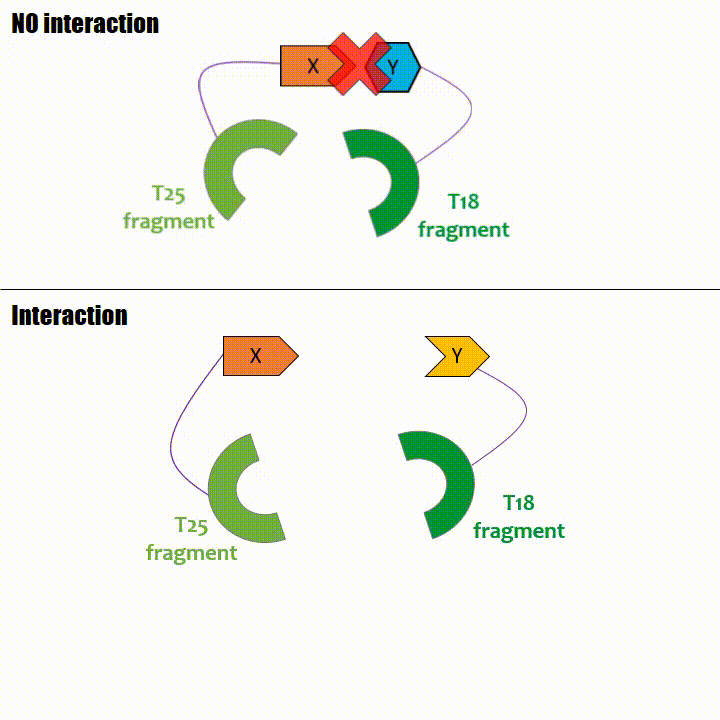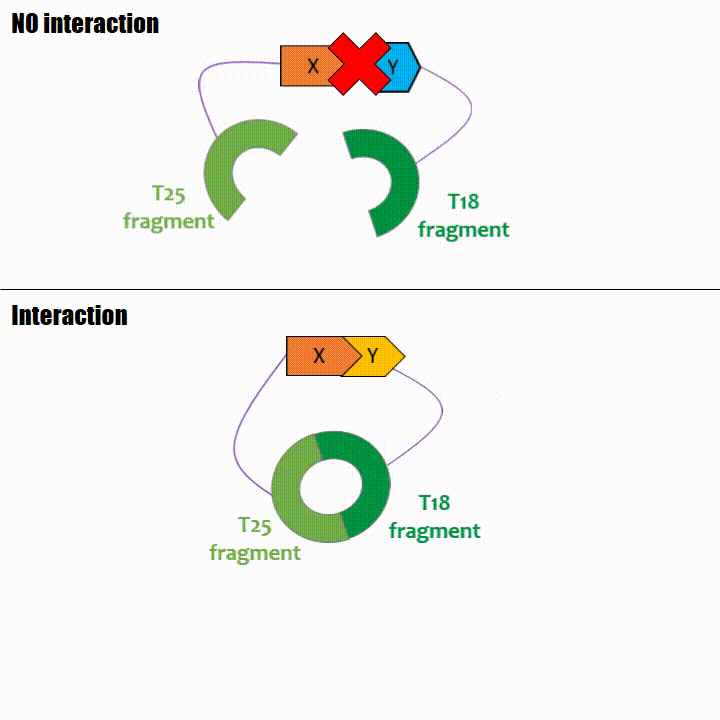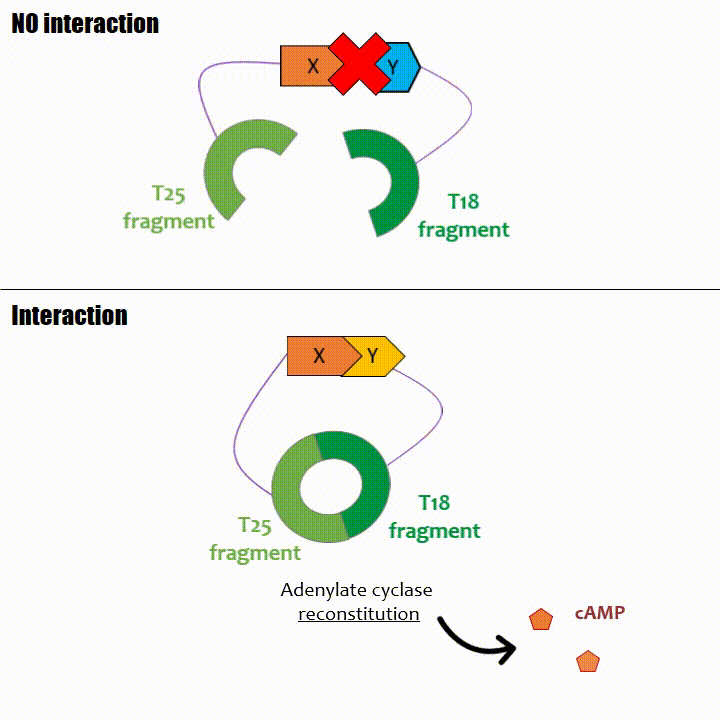
When two proteins interact, then T18 and T25 are bring together and reconstitute a functional adenylate cyclase enzyme thus enabling cAMP production. Using
cya- bacteria – strain for whom the adenylate gene is deleted, involving an absence of this endogenous enzyme – a BACTH could be done with the creation of two
fusion proteins : the first one, fused at its N or C terminal intracellular end with the T18 sub-part; the second one fused with the T25 sub-part.
The interaction of these proteins of interest will lead to the adenylate cyclase reconstitution, thus initiating cAMP production. The cAMP produced will act as a
messenger by fixing itself to the transcriptional activator CAP, cAMP form the CAP-cAMP complex, controlling the expression of the lactose promoter by initiating transcription of the following gene.
This promoter is placed upstream the chosen reporter gene.
NeuroDrop detection system is based on the use of a BACTH. The point is to allow the induction of the gene only when the two sub-parts of AC are physically close, which only occurs when the target is present in the sample. The reassembly of AC enables cAMP production, followed by the transcription of the reporter that is under a CAP-cAMP dependent promoter.
To use this system, an AC deficient bacteria strain (BTH101) that is not able to produce endogenous cAMP is needed to prevent any transcription from CAP dependant promoter in abscence of interaction between both sub-parts.
For the promoter, we decided to use the lactose promoter (a CAP dependent promoter), we have demonstrated its repression in the absence of an exogenous source of cAMP (in the AC deficient bacterial strain).
References
Fields S, Song O. A novel genetic system to detect protein–protein interactions. Nature. (1989)
Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. PNAS. (1998)
Karimova G, Gauliard E, Davi M, P.Ouellette S, Ladant D. Protein–Protein Interaction: Bacterial Two-Hybrid. (2017)
Picture of the reaction ATP-cAMP. Khan Academy Website. Retrieved October 10, 2019, from https://www.khanacademy.org
Euromedex, BACTH System Kit
Leusch, Paulaitis, Friedman. Adenylate cyclase toxin of Bordetella pertussis: production, purification, and partial characterization. Am Soc Microbiol | Infect Immun. (1990)
Hantke, Winkler, Schultz. Escherichia coli exports cyclic AMP via TolC. J Bacteriol. (2011)
