Difference between revisions of "Part:BBa K3257100"
| (5 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
This part is the system we build to express our improved LacI. With lacIq promoter and rrnB T1 terminator, improved LacI protein can be expressed and function properly in the Escherichia coli BL21(DE3). We used EGFP as a reporter controlled by our improved Lac operon and measured its green fluorescence over time. | This part is the system we build to express our improved LacI. With lacIq promoter and rrnB T1 terminator, improved LacI protein can be expressed and function properly in the Escherichia coli BL21(DE3). We used EGFP as a reporter controlled by our improved Lac operon and measured its green fluorescence over time. | ||
| − | According to our experiment, our | + | According to our experiment, our Lac operon is improved in the following three main aspects. |
| − | '''Lower Response to Arabinose''' | + | '''Lower Response to Arabinose, Better Orthogonality''' |
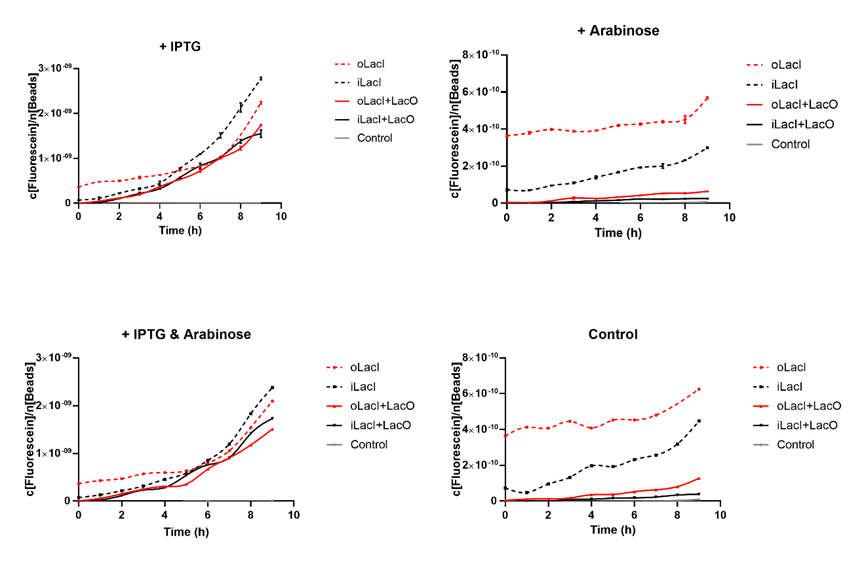
| − | ''' | + | Cross talk between the response to IPTG and arabinose has been a defect of the wild type Lac operon. When 4mM arabinose added, a few lac inhibitors detach from lac operator which means that it is induced in a relatively low but unignorable level. According to the measurement of our experiment, our improved LacI can respond to IPTG with better orthogonality. The figure below (Figure 1) is the measurement of the fluorescence of EGFP controlled by wild-type and improved Lac operon. It shows that when 4mM arabinose is added, oLacI(wild-type LacI) is induced at a significantly high level while iLacI(improved LacI) is induced at a lower level. |
| + | |||
| + | [[File:ILacI Curves.png|center|500px|thumb|'''Figure 1. The expression level of EGFP controlled by different versions of LacI and inducers, or under different promoters.'''The origin point indicates the time when different inducers are added (1 mM IPTG and/or 4 mM Arabinose). The title of the graph shows which kind of inducer is added to the culture. The horizontal axis shows the duration of time, the vertical axis shows the quantified level of EGFP expression. The fluorescence level (excitation wavelength: 485 nm; detection wavelength: 528 nm) is quantified by the concentration of fluorescein, and normalized by the measured OD600 equivalent to the number of beads in the system. oLacI stands for the wildtype LacI, iLacI stands for our improved version of LacI. +LacO indicates that the promoter constitutes a LacO sequence. Control is the negative control plasmid which does not constitute an EGFP sequence. Error bar in the two graphs on the first row indicates the SEM of three replicates. The second row showed only the mean amount of three replicate.]] | ||
| + | |||
| + | '''Higher Induction Level than the Wild-type Lac operon when Induced by IPTG''' | ||
| + | |||
| + | Next step we prove that our improved Lac operon can be normally induced by IPTG, which counts a great deal for the usage of this operon. The figure below (Figure 2) is the measurement of the fluorescence of EGFP controlled by wild-type and improved Lac operon. It shows that when 1mM IPTG is added, EGFP is better induced when it is controlled by the improved Lac operon. | ||
| + | |||
| + | [[File:ILacI+IPTG.png|center|500px|thumb|'''Figure 2. The induction level of EGFP under different repressors and promoters.''' The induction level is calculated by dividing the fluorescence level after 9 h of induction by 1 h afterward. The fluorescence level is quantified as in Fig. 1. oLacI stands for the wildtype LacI, iLacI stands for our improved version of LacI. +LacO indicates that the promoter constitutes a LacO sequence. t-test analysis shows that the induction level of iLacI is significantly higher than oLacI, *** indicates that p=0.0002.]] | ||
'''Lower Uninduced Leakage''' | '''Lower Uninduced Leakage''' | ||
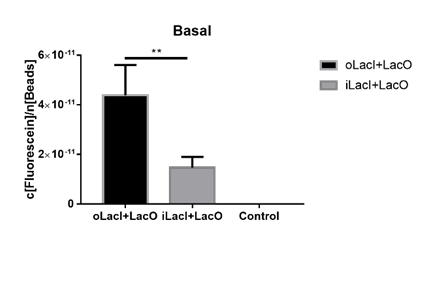
| + | The uninduced leakage level is also an important parameter of an operon. Improved LacI lowers the leakage level compared to the wild-type one. The figure below (Figure 3) is the measurement of the fluorescence of EGFP controlled by wild-type and improved Lac operon. When no IPTG or arabinose is added, the fluorescence of EGFP controlled by improved Lac operon is under the detection range while the fluorescence of EGFP controlled by wild-type Lac operon remains a detectable signal indicating a considerably undesired leakage. | ||
| + | |||
| + | [[File:ILacI uninduced.png|center|500px|thumb|'''Figure 3. The basal fluorescence level of EGFP controlled by different repressors.''' The bar indicates the mean fluorescence level during the 10 h with no inducer in the culture. The fluorescence level is quantified as in Fig. 1. oLacI stands for the wildtype LacI, iLacI stands for our improved version of LacI. Control is below the detection level and not shown. Error bar indicates the SEM of fluorescence signal in the 10 h. Paired t-test analysis shows that iLacI has a significantly lower of fluorescence than oLacI, p=0.0057 (**).]] | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Latest revision as of 02:48, 18 October 2019
Improved LacI Expression System
This part is the system we build to express our improved LacI. With lacIq promoter and rrnB T1 terminator, improved LacI protein can be expressed and function properly in the Escherichia coli BL21(DE3). We used EGFP as a reporter controlled by our improved Lac operon and measured its green fluorescence over time.
According to our experiment, our Lac operon is improved in the following three main aspects.
Lower Response to Arabinose, Better Orthogonality
Cross talk between the response to IPTG and arabinose has been a defect of the wild type Lac operon. When 4mM arabinose added, a few lac inhibitors detach from lac operator which means that it is induced in a relatively low but unignorable level. According to the measurement of our experiment, our improved LacI can respond to IPTG with better orthogonality. The figure below (Figure 1) is the measurement of the fluorescence of EGFP controlled by wild-type and improved Lac operon. It shows that when 4mM arabinose is added, oLacI(wild-type LacI) is induced at a significantly high level while iLacI(improved LacI) is induced at a lower level.
Higher Induction Level than the Wild-type Lac operon when Induced by IPTG
Next step we prove that our improved Lac operon can be normally induced by IPTG, which counts a great deal for the usage of this operon. The figure below (Figure 2) is the measurement of the fluorescence of EGFP controlled by wild-type and improved Lac operon. It shows that when 1mM IPTG is added, EGFP is better induced when it is controlled by the improved Lac operon.

Lower Uninduced Leakage
The uninduced leakage level is also an important parameter of an operon. Improved LacI lowers the leakage level compared to the wild-type one. The figure below (Figure 3) is the measurement of the fluorescence of EGFP controlled by wild-type and improved Lac operon. When no IPTG or arabinose is added, the fluorescence of EGFP controlled by improved Lac operon is under the detection range while the fluorescence of EGFP controlled by wild-type Lac operon remains a detectable signal indicating a considerably undesired leakage.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal XbaI site found at 1258
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23INCOMPATIBLE WITH RFC[23]Illegal XbaI site found at 1258
- 25INCOMPATIBLE WITH RFC[25]Illegal XbaI site found at 1258
- 1000COMPATIBLE WITH RFC[1000]
Reference
AJ Meyer et al. Escherichia coli "Marionette" strains with 12 highly optimized small-molecule sensors. Nat Chem Biol. 2019 Feb;15(2):196-204. doi: 10.1038/s41589-018-0168-3. Epub 2018 Nov 26.
