Difference between revisions of "Part:BBa K2571003"
(→Biochemical Characterization) |
|||
| (3 intermediate revisions by the same user not shown) | |||
| Line 91: | Line 91: | ||
VR: ATTACCGCCTTTGAGTGAGC | VR: ATTACCGCCTTTGAGTGAGC | ||
| + | |||
| + | |||
| + | |||
=== Biochemical Characterization: === | === Biochemical Characterization: === | ||
| + | |||
We designed our biochemical characterization experiments in order to evaluate the effects of our circuits on life span, cell mass, and ultimately the bioethanol yield of ethanologenic E. coli strain KO11. We carried out two experimental assays simultaneously. | We designed our biochemical characterization experiments in order to evaluate the effects of our circuits on life span, cell mass, and ultimately the bioethanol yield of ethanologenic E. coli strain KO11. We carried out two experimental assays simultaneously. | ||
| Line 98: | Line 102: | ||
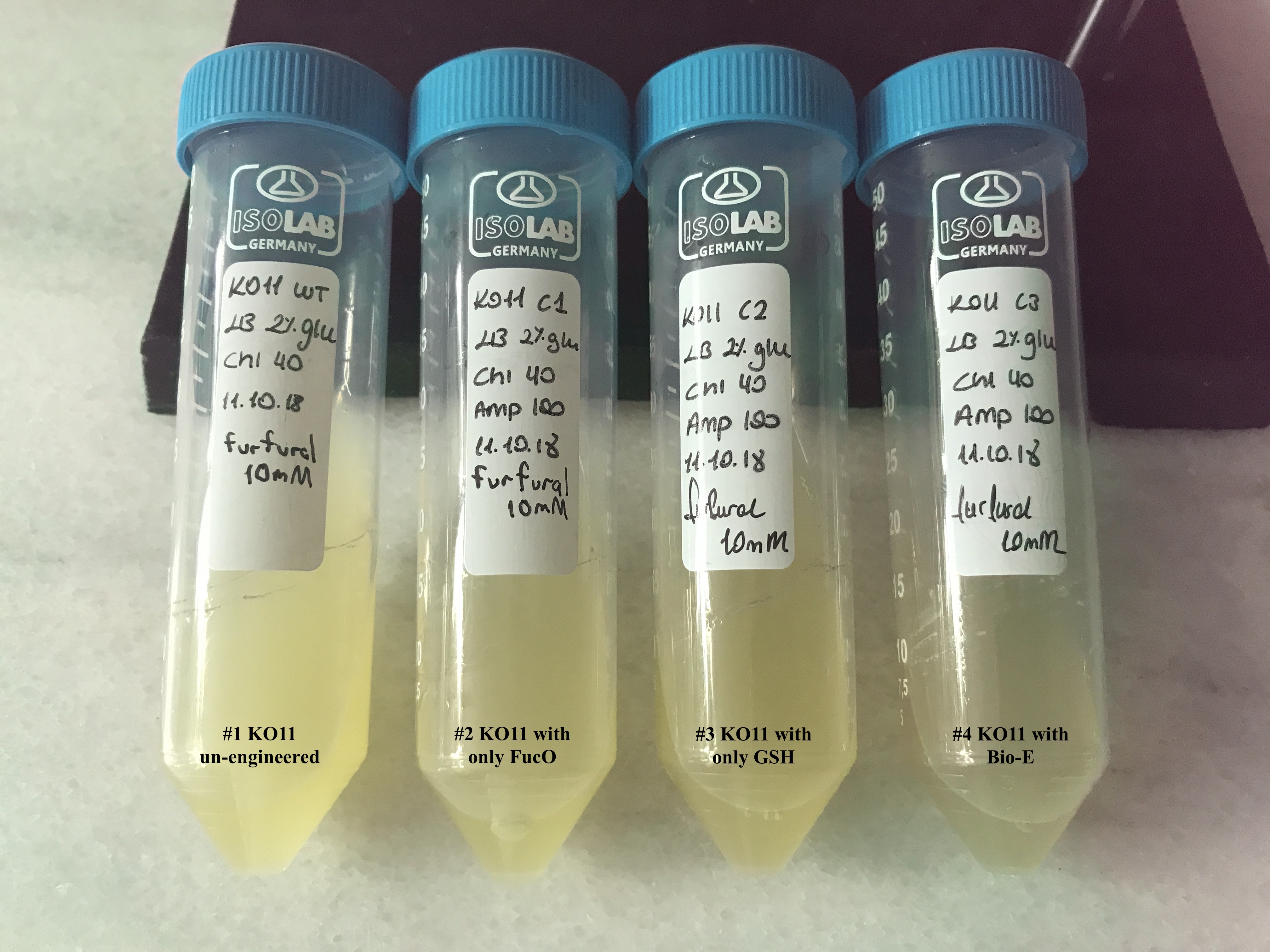
In both of our biochemical assays, we had four cultured groups of KO11 ethanologenic strains of E.coli to test. | In both of our biochemical assays, we had four cultured groups of KO11 ethanologenic strains of E.coli to test. | ||
| − | 1 KO11 un-engineered | + | |
| − | 2 KO11 with only FucO | + | 1. KO11 un-engineered, |
| − | 3 KO11 with only GSH | + | 2. KO11 with only FucO, |
| − | 4 KO11 with Bio-E (FucO and GSH) | + | 3. KO11 with only GSH, |
| + | 4. KO11 with Bio-E (both FucO and GSH). | ||
| + | |||
| Line 146: | Line 152: | ||
<b>Analysis of data:</b> | <b>Analysis of data:</b> | ||
| − | Our data demonstrated that group #1 (un-engineered) had a decrease in cell mass throughout the time verifying the inhibition of cell growth in the presence of furfural in the field. Group #2 (KO11 with only FucO) obviously gave better results with respect to un-engineered KO11. However, although the cell mass of the KO11 group with only FucO was stable in the first 48 hours, it was decreased after the 48th hour. This proves that only the presence of the gene FucO in the bacteria wasn’t enough to avoid cell mass decrease in the long term and is in need of another gene for increased tolerance. Also, only GSH’s presence isn’t enough since the group of KO11 with only GSH experienced decrease in cell mass. Group #4 (KO11 with both FucO and GSH) gave measurement results as we hypothesized by continuing cellular growth in the first 48 hours and maintaining it even after the 48th hour, though at a lower rate. | + | Our data demonstrated that group #1 (un-engineered) had a decrease in cell mass throughout the time verifying the inhibition of cell growth in the presence of furfural in the field. Group #2 (KO11 with only FucO) obviously gave better results with respect to un-engineered KO11. However, although the cell mass of the KO11 group with only FucO was stable in the first 48 hours, it was decreased after the 48th hour. This proves that only the presence of the gene FucO in the bacteria wasn’t enough to avoid cell mass decrease in the long term and is in need of another gene for increased tolerance. Also, only GSH’s presence isn’t enough since the group of KO11 with only GSH experienced decrease in cell mass. Group #4 (KO11 with Bio-E (both FucO and GSH)) gave measurement results as we hypothesized by continuing cellular growth in the first 48 hours and maintaining it even after the 48th hour, though at a lower rate. |
| Line 161: | Line 167: | ||
To gather more information to prove our hypothesis, we designed our second experimental set and added furfural at a final concentration of 20 mM to the four test groups’ mediums followed by OD measurements at Abs 600 nm with 1/10 dilution in 24 hour time intervals. | To gather more information to prove our hypothesis, we designed our second experimental set and added furfural at a final concentration of 20 mM to the four test groups’ mediums followed by OD measurements at Abs 600 nm with 1/10 dilution in 24 hour time intervals. | ||
| − | 20 mM furfural OD measurements: Abs 600 (1/10 dilution) | + | 20 mM furfural OD measurements: Abs 600 (1/10 dilution): |
| Line 180: | Line 186: | ||
<b> Analysis of Data: </b> | <b> Analysis of Data: </b> | ||
| − | Our data demonstrated that group #1 (KO11 un-engineered) had a decrease in cell mass as time passed. Group #2 (KO11 with only FucO) also experienced a decrease in cell mass. Group #3 (KO11 with only GSH) gave better results with respect to both of the groups #1 and #2 by maintaining its cell mass stable. Group #4 (KO11 with both FucO and GSH) gave the most promising measurement data as we hypothesized by continuing cellular growth (almost doubling cell mass) in the first 48 hours. | + | Our data demonstrated that group #1 (KO11 un-engineered) had a decrease in cell mass as time passed. Group #2 (KO11 with only FucO) also experienced a decrease in cell mass. Group #3 (KO11 with only GSH) gave better results with respect to both of the groups #1 and #2 by maintaining its cell mass stable. Group #4 (KO11 with Bio-E (both FucO and GSH)) gave the most promising measurement data as we hypothesized by continuing cellular growth (almost doubling cell mass) in the first 48 hours. |
| Line 213: | Line 219: | ||
| − | [[File:T--METU HS Ankara assay resim 5 plate.jpg|350px|thumb|right|Plate having group KO11 with FucO and GSH.]] | + | [[File:T--METU HS Ankara assay resim 5 plate.jpg|350px|thumb|right|Plate having group KO11 with Bio-E (FucO and GSH.)]] |
| Line 276: | Line 282: | ||
When the quantitative measurement data and qualitative phenotypic evaluation for all of our biochemical assays are considered, we can conclude that the groups containing KO11 un-engineered are the weakest ones against furfural toxicity; and neither the group of KO11 with only FucO nor the group with only GSH is resistant enough to continue cellular growth when furfural is present in the medium. Out of four groups, only the group containing KO11 with Bio-E (both FucO and GSH) can sustain its cellular growth and survive. Overall, we can infer that our best part design (Bio-E) was successful enough to combat the inhibitive effects of furfural, indicating trueness of our hypothesis. | When the quantitative measurement data and qualitative phenotypic evaluation for all of our biochemical assays are considered, we can conclude that the groups containing KO11 un-engineered are the weakest ones against furfural toxicity; and neither the group of KO11 with only FucO nor the group with only GSH is resistant enough to continue cellular growth when furfural is present in the medium. Out of four groups, only the group containing KO11 with Bio-E (both FucO and GSH) can sustain its cellular growth and survive. Overall, we can infer that our best part design (Bio-E) was successful enough to combat the inhibitive effects of furfural, indicating trueness of our hypothesis. | ||
| − | |||
| Line 282: | Line 287: | ||
<!-- Add more about the biology of this part here --> | <!-- Add more about the biology of this part here --> | ||
| + | |||
| + | |||
| + | |||
=== References === | === References === | ||
Latest revision as of 19:52, 17 October 2018
FucO L-1,2-propanediol oxidoreductase
Usage and Biology
L-1,2-propanediol oxidoreductase is an iron-dependent group III dehydrogenase.
FucO is the gene that codes for L-1,2-propanediol oxidoreductase which is a NADH-linked, homodimer enzyme having the role of acting on furfural which is a toxic inhibitor of microbial fermentations causing cell wall and membrane damage, DNA breaks down and reduced enzymatic activities (Zheng, 2013; Liu, Ma & Song, 2009).
The enzyme catalyzes L-lactaldehyde and L-1,2- propanediol while dissimilating fucose in which acetaldehyde, ethylene glycerol, L-lactaldehyde and some more substances are used as substrates. Despite these, it takes an important role in furan reduction to its alcohol derivative (Wang et al. , 2011).
Source:
Gene : FucO
Protein : L-1,2-Propanediol Oxidoreductase
Organism : E.coli K12 (MG 1665)
Design Notes of FucO (BBa_K2571003)
Our circuit consists of prefix, a strong promoter (J23100), RBS (B0034), FucO as protein coding region, double terminator (B0015) and suffix. This part enables our E. coli KO11 strain to convert toxic furfural into furfuryl alcohol. Our construct is inserted into pSB1C3 and delivered to the Registry.
In order to make our gene compatible with RFC 10, 25 and 1000, we reconstructed the nucleotides to get rid of the restriction sites while protecting the amino acid sequence. We looked through the codon bias property of E. coli and made the nucleotide changes accordingly.
FucO has NADH-dependent furan reductase activity. When furfural is present in the field, the metabolism of furfural by NADPH-dependent oxidoreductases go active in order to reduce it to its less toxic alcohol derivative-furfuryl alcohol (Zheng, 2013; Wang et al., 2013; Allen et al., 2010).

In this metabolism, the expression of oxidoreductases that are NADPH-dependent, such as YqhD, are shown to inhibit the growth and fermentation in E. coli by competing for biosynthesis with NADPH (Zheng, 2013).
Because the native conversion of NADH to NADPH in E. coli is insufficient to revitalize the NADPH pool during fermentation, the actions shouldn’t be interfering with NADPH metabolism (Wang et al. , 2011). Thus, the overexpression of plasmid-based NADH-dependent propanediol oxidoreductase (FucO) gene reduces furfural to ultimately improve furfural resistance without detrimentally affecting the biosynthesis of NADPH (Wang et al., 2011).
Figure represents the predicted three-dimensional structure of 1,2-propanediol oxidoreductase from E. coli . The protein structure of L-1,2-propanediol oxidoreductase was constructed by using Amber 14. It is demonstrated in the ribbon diagram which is done by interpolating a smooth curve through the polypeptide backbone. The colors indicate the amino acids in the protein structure. While constructing, the codon bias rule is obeyed to express the enzyme in Escherichia Coli KO11.
Characterization
Allergen Characterization:
Our parts can be used in ethanol production and we used it in the lab for mass production, it was important to construct an allergenicity test. The allergenicity test makes a comparison between the sequences of the biobrick parts and the identified allergen proteins in the database. If the similarity between the biobricks and the proteins is high, it is more likely that the biobrick is allergenic. In the sliding window of 80 amino acid segments, greater than 35% means similarity to allergens. Higher similarity implies that the biobricks have a potential for negative effect to exposed populations. For more information on the protocol see the “Allergenicity Testing Protocol” in the following page http://2017.igem.org/Team:Baltimore_Bio-Crew/Experiments
Our biobrick part, BBa_K2571003 showed less than 35% match in the 80 amino acid alignments by FASTA.
Gel Characterization:
FucO composite part is inserted into the pSB1C3 backbone. The construct in pSB1C3 is for submission to the registry and is cultivated in DH5 alpha.
We’ve inserted the FucO composite part to pSB1C3 and pSB1A3 backbones. Then, we’ve transformed the construct for submission, BBa_K2571003, (in pSB1C3) to DH5 alpha; and the other construct, for our biochemical assay, (in pSB1A3) to KO11. As we isolated the plasmids, we’ve done PCR with FucO left and VR primers to test orientation of our parts to the backbone. We expected a band of 754 bp between the FucO left and VR primers and the PCR results confirmed our expectations and showed that our parts were correctly inserted and transformed.
FucO and VR primers are as below:
FucO left: GTGATAAGGATGCCGGAGAA
VR: ATTACCGCCTTTGAGTGAGC
Biochemical Characterization:
We designed our biochemical characterization experiments in order to evaluate the effects of our circuits on life span, cell mass, and ultimately the bioethanol yield of ethanologenic E. coli strain KO11. We carried out two experimental assays simultaneously.
In both of our biochemical assays, we had four cultured groups of KO11 ethanologenic strains of E.coli to test.
1. KO11 un-engineered,
2. KO11 with only FucO,
3. KO11 with only GSH,
4. KO11 with Bio-E (both FucO and GSH).
First Assay:
Throughout our first assay, each group was grown in LB broth mediums containing 2% glucose and antibiotics.
To culture group #1 (KO11 un-engineered), we only added Chloramphenicol at a final concentration of 40 µg/mL since KO11 un-engineered only had resistance to Chloramphenicol in its genome; and to the mediums of the groups numbered 2, 3 and 4; we added Chloramphenicol at a final concentration of 40 µg/ml and 100 µg/ml Ampicillin. The reason was; groups 2, 3 and 4 had plasmids which carried Ampicillin resistance due to their backbone (pSB1A3). Thus, with the addition of antibiotics to the mediums, selectivity was assured.
Cultures were grown overnight, and refreshed in the morning as two sets (First set: 10 mM furfural, 2nd set: 20 mM furfural). After approximately two hours of incubation for both of the sets’ falcon groups (when they reached OD 0,6), furfural was added to their mediums.
First Set (10 mM furfural):
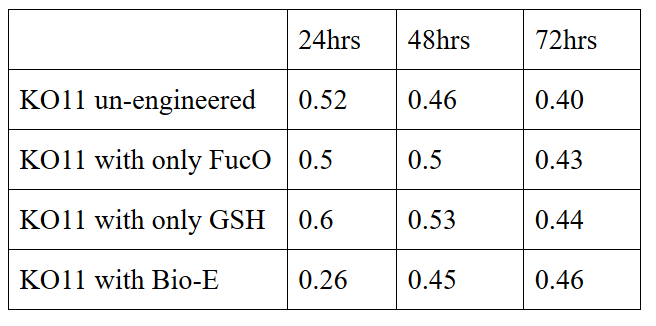
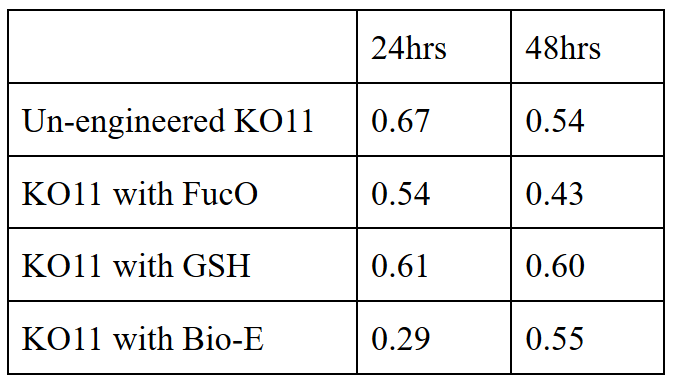
We added furfural at a final concentration of 10 mM to the first four test groups’ mediums and took OD measurements at Abs 600 nm with 1/10 dilution in 24 hour time intervals.
10 mM furfural OD measurements: Abs 600 nm (1/10 dilution):
Analysis of data:
Our data demonstrated that group #1 (un-engineered) had a decrease in cell mass throughout the time verifying the inhibition of cell growth in the presence of furfural in the field. Group #2 (KO11 with only FucO) obviously gave better results with respect to un-engineered KO11. However, although the cell mass of the KO11 group with only FucO was stable in the first 48 hours, it was decreased after the 48th hour. This proves that only the presence of the gene FucO in the bacteria wasn’t enough to avoid cell mass decrease in the long term and is in need of another gene for increased tolerance. Also, only GSH’s presence isn’t enough since the group of KO11 with only GSH experienced decrease in cell mass. Group #4 (KO11 with Bio-E (both FucO and GSH)) gave measurement results as we hypothesized by continuing cellular growth in the first 48 hours and maintaining it even after the 48th hour, though at a lower rate.
Second Set (20 mM furfural):
To gather more information to prove our hypothesis, we designed our second experimental set and added furfural at a final concentration of 20 mM to the four test groups’ mediums followed by OD measurements at Abs 600 nm with 1/10 dilution in 24 hour time intervals.
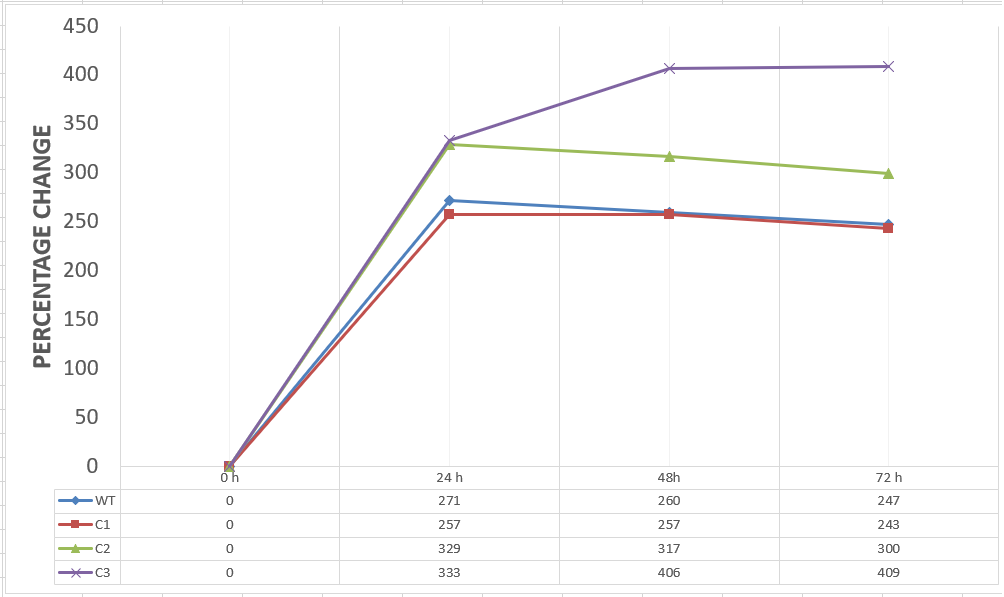
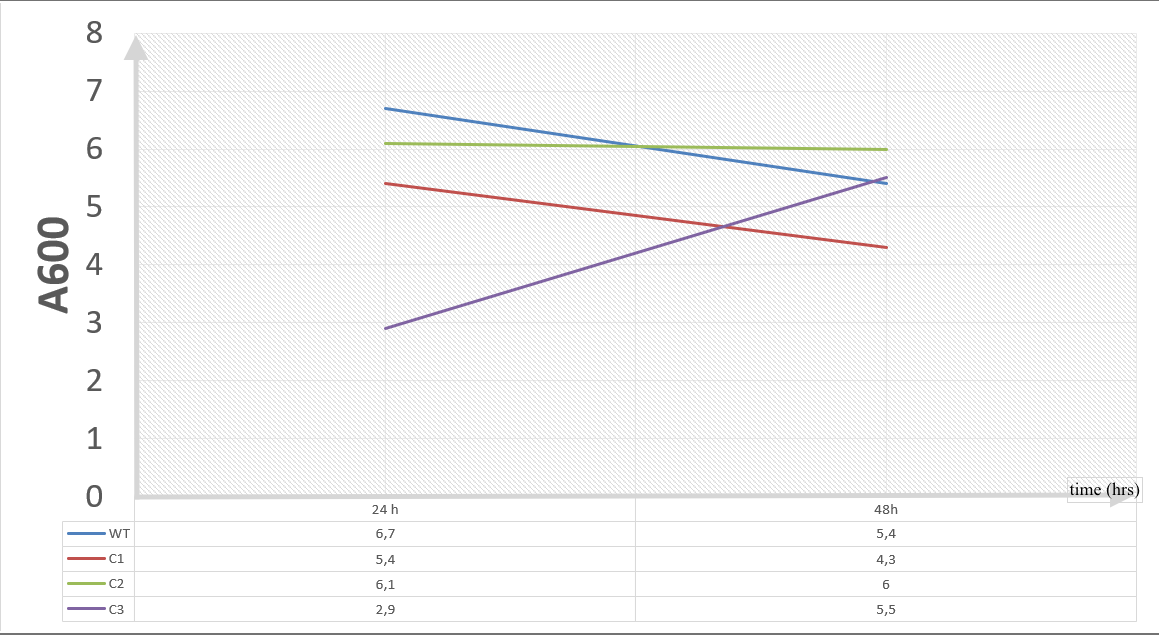
20 mM furfural OD measurements: Abs 600 (1/10 dilution):
For our second set, we could only obtain the measurements of the first 48 hours since we faced contamination in the last day of wet-lab and we had no more time. Thus, we modelled our second experimental set’s results by demonstrating the comparison of OD results at absorbance 600.
Analysis of Data:
Our data demonstrated that group #1 (KO11 un-engineered) had a decrease in cell mass as time passed. Group #2 (KO11 with only FucO) also experienced a decrease in cell mass. Group #3 (KO11 with only GSH) gave better results with respect to both of the groups #1 and #2 by maintaining its cell mass stable. Group #4 (KO11 with Bio-E (both FucO and GSH)) gave the most promising measurement data as we hypothesized by continuing cellular growth (almost doubling cell mass) in the first 48 hours.
Second Assay:
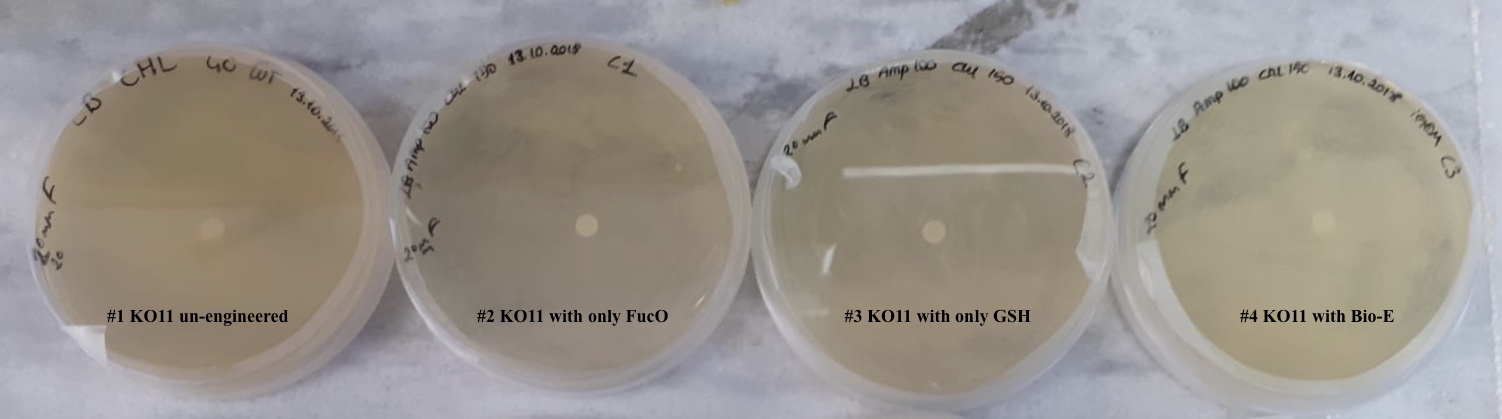
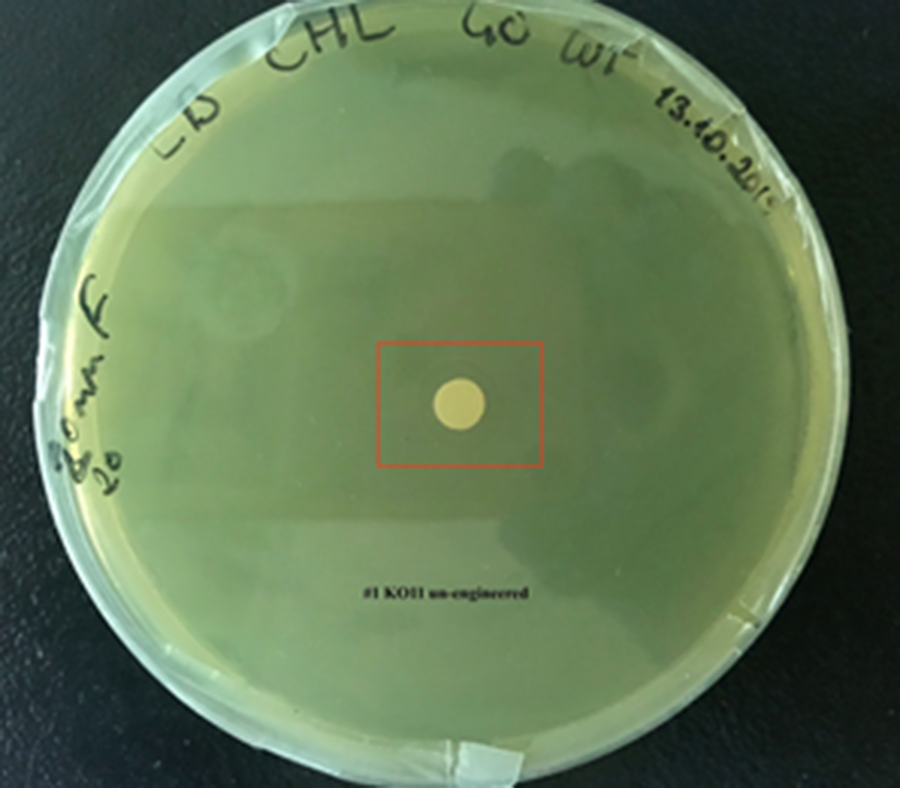
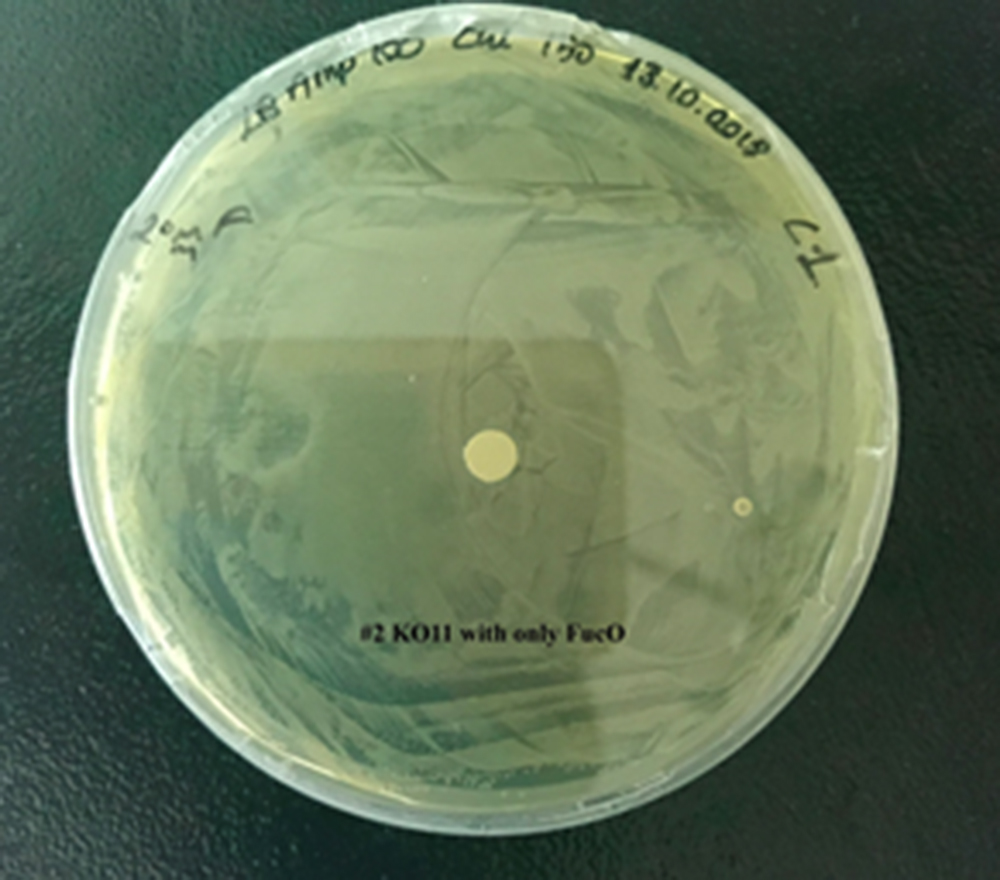
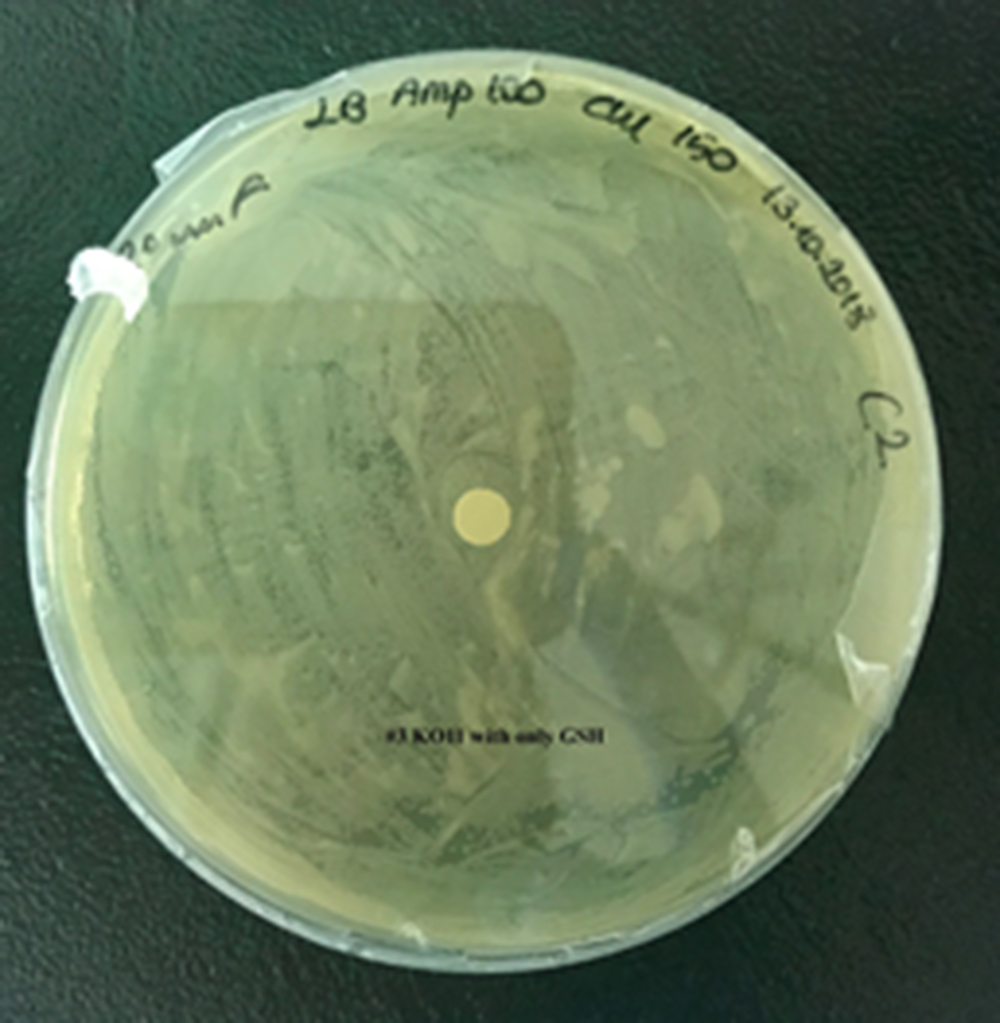
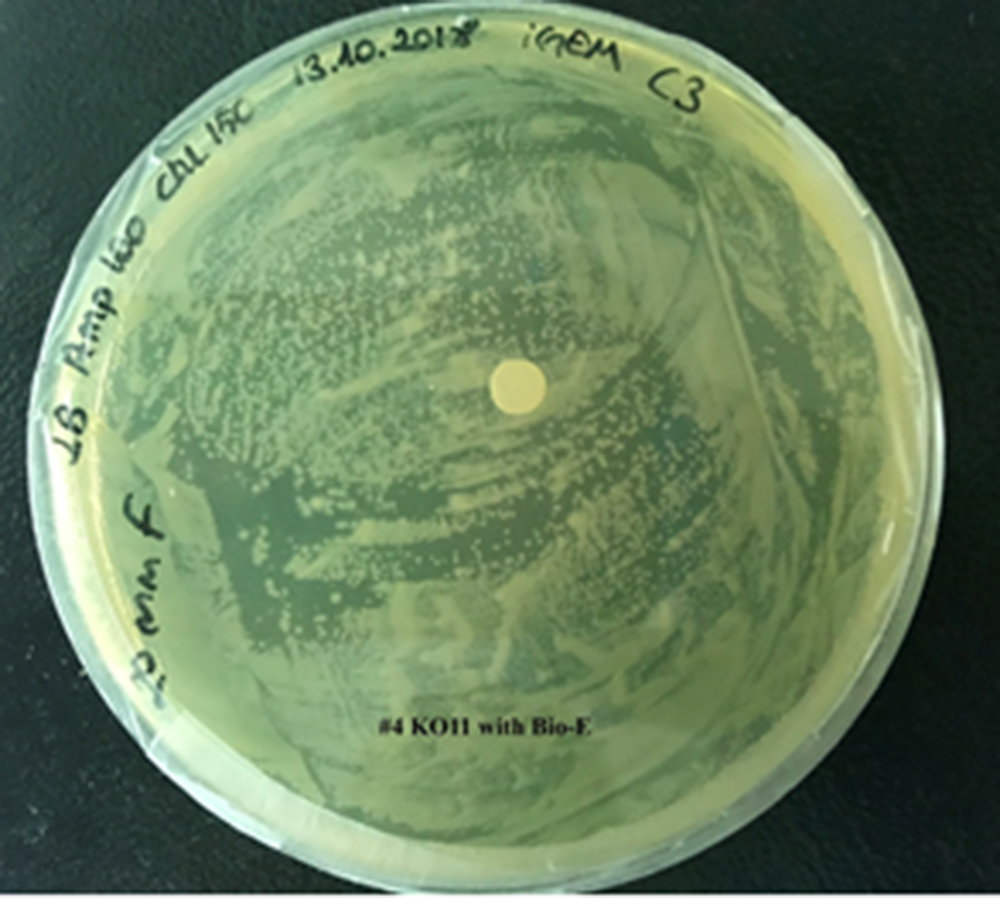
For our second characterization, we followed more of qualitative evaluation to see the inhibitory zone of furfural. Firstly, we prepared a solution of furfural at a final concentration of 20 mM by diluting the stock solution with distilled H2O. Then, we soaked filter paper discs in that solution and placed them on LB agar plates hosting the four groups of our assay.
After 48 hours, we’ve observed a clear zone around the filter paper of the group containing KO11 un-engineered while others weren’t inhibited as much.
Conclusion:
When the quantitative measurement data and qualitative phenotypic evaluation for all of our biochemical assays are considered, we can conclude that the groups containing KO11 un-engineered are the weakest ones against furfural toxicity; and neither the group of KO11 with only FucO nor the group with only GSH is resistant enough to continue cellular growth when furfural is present in the medium. Out of four groups, only the group containing KO11 with Bio-E (both FucO and GSH) can sustain its cellular growth and survive. Overall, we can infer that our best part design (Bio-E) was successful enough to combat the inhibitive effects of furfural, indicating trueness of our hypothesis.
References
Allen, S. A., Clark, W., McCaffery, J. M., Cai, Z., Lanctot, A., Slininger, P. J., … Gorsich, S. W. (2010). Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnology for Biofuels, 3, 2. http://doi.org/10.1186/1754-6834-3-2
Chou, H.-H., Marx, C. J., & Sauer, U. (2015). Transhydrogenase Promotes the Robustness and Evolvability of E. coli Deficient in NADPH Production. PLoS Genetics, 11(2), e1005007. http://doi.org/10.1371/journal.pgen.1005007
Liu, Z.L., Ma M., Song, M.(2009). Evolutionarily engineered ethanologenic yeast detoxifies lignocellulosic biomass conversion inhibitors by reprogrammed pathways. Mol Genet Genomics 282, 233-244. doi: 10.1007/s00438-009-0461-7
Zheng, H., Wang, X., Yomano, L.P., Geddes, R. D, Shanmugan, K. T., Ingram, L.O. (2013). Improving Escherichia coli FucO for Furfural Tolerance by Saturation Mutagenesis of Individual Amino Acid Positions. Applied and Environmental Microbiology Vol 79, no 10. 3202–3208. http://aem.asm.org/content/79/10/3202.full.pdf+html
Wang, X., Miller, E. N., Yomano, L. P., Zhang, X., Shanmugam, K. T., & Ingram, L. O. (2011). Increased Furfural Tolerance Due to Overexpression of NADH-Dependent Oxidoreductase FucO in Escherichia coli Strains Engineered for the Production of Ethanol and Lactate. Applied and Environmental Microbiology, 77(15), 5132–5140. http://doi.org/10.1128/AEM.05008-11
Wang, X., Yomano, L. P., Lee, J. Y., York, S. W., Zheng, H., Mullinnix, M. T., … Ingram, L. O. (2013). Engineering furfural tolerance in Escherichia coli improves the fermentation of lignocellulosic sugars into renewable chemicals. Proceedings of the National Academy of Sciences of the United States of America, 110(10), 4021–4026. http://doi.org/10.1073/pnas.1217958110
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]