Difference between revisions of "Part:BBa K2796028"
(→Experimental results) |
Ahmed Mattar (Talk | contribs) (→Characterization of the improved part by AFCM-Egypt 2023 team) |
||
| (16 intermediate revisions by 4 users not shown) | |||
| Line 37: | Line 37: | ||
[[File:2018 LZU-CHINA model6.png|300px|thumb|center|]] | [[File:2018 LZU-CHINA model6.png|300px|thumb|center|]] | ||
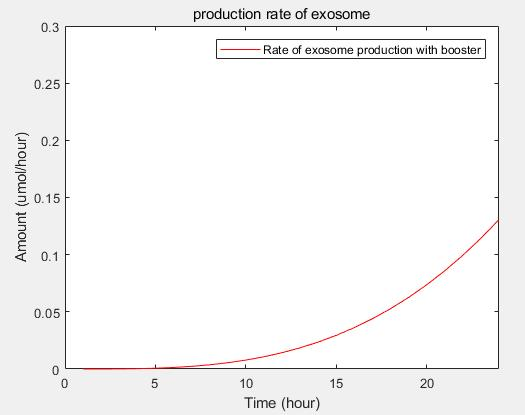
This model predicts the production rate of exosome over time in cells. | This model predicts the production rate of exosome over time in cells. | ||
| + | ==Improvement by AFCM-Egypt 2023 team== | ||
| + | This composite part is an improvement for (BBa_K2796028) Designed by (iGEM18_LZU-CHINA).We developed this new approach to control the expression of these booster genes (SDC4, STEAP3, NadB) that enhance the exosomes secretion within our engineered mesenchymal stem cell, as we found out that constitutive overexpression of these genes may carry out multiple side effect in addition to depleting MSCs resources and impairing its function and survival, so we implemented the Cre loxP system for conditional gene expression to induce the expression of these booster genes by deleting (STOP) sequence that prevents the transcription of the downstream genes. Thus, the expression of booster genes as condition in the presence of Cre recombinase enzyme after vimentin syn notch activation. | ||
| + | <html><div align="center"style="border:solid #17252A; width:80%;float:center;"><img style=" max-width:850px; | ||
| + | width:100%; | ||
| + | height:auto; | ||
| + | position: relative; | ||
| + | top: 50%; | ||
| + | left: 50%; | ||
| + | transform: translate( -50%); | ||
| + | padding-bottom:25px; | ||
| + | padding-top:25px; | ||
| + | "src="https://static.igem.wiki/teams/4586/wiki/parts/picture2.png"> | ||
| + | <p class=MsoNormal align=center style='text-align:left;border:none;width:98% ;justify-content:center;'><span | ||
| + | lang=EN style='font-size:11.0pt;line-height:115%'>This figure illustrates the conditional expression of our enhanced booster genes that increase the exosomal secretion. | ||
| + | </span></p></div></html> | ||
| + | Presence of SDC4 as a booster gene has a role in increasing the concentration of the produced engineered exosomes so it plays an effective role to increase the efficacy of the therapeutic agent. | ||
| + | |||
| + | We compared both condition of exosomes production when using booster genes and without it | ||
| + | <br><br> | ||
| + | (1)No booster genes with conditioned release | ||
| + | <html><div align="center"style="border:solid #17252A; width:100%;float:center;"><img style=" max-width:850px; | ||
| + | width:100%; | ||
| + | height:auto; | ||
| + | position: relative; | ||
| + | top: 50%; | ||
| + | left: 45%; | ||
| + | transform: translate( -50%); | ||
| + | padding-bottom:25px; | ||
| + | padding-top:25px; | ||
| + | "src="https://static.igem.wiki/teams/4586/wiki/modeling/no-booster.png"> | ||
| + | <p class=MsoNormal align=center style='text-align:left;border:none;width:98% ;justify-content:center;'><span | ||
| + | lang=EN style='font-size:11.0pt;line-height:115%'>This Represents the relation between the activation of the internal domain of the syn-noth (represented as red line) and production of exosomes with specific cargo (represented as blue line) as the production of the engineered exosomes is initiated once the internal domain is activated. | ||
| + | </span></p></div></html> | ||
| + | <br><br> | ||
| + | (2)Booster gene with conditioned release | ||
| + | <html><div align="center"style="border:solid #17252A; width:100%;float:center;"><img style=" max-width:850px; | ||
| + | width:100%; | ||
| + | height:auto; | ||
| + | position: relative; | ||
| + | top: 50%; | ||
| + | left: 45%; | ||
| + | transform: translate( -50%); | ||
| + | padding-bottom:25px; | ||
| + | padding-top:25px; | ||
| + | "src="https://static.igem.wiki/teams/4586/wiki/modeling/booster.png"> | ||
| + | <p class=MsoNormal align=center style='text-align:left;border:none;width:98% ;justify-content:center;'><span | ||
| + | lang=EN style='font-size:11.0pt;line-height:115%'>This Represents the relation between the activation of the internal domain of the syn-noth (represented as red line) and production of exosomes with specific cargo (represented as blue line) as the production of the engineered exosomes is initiated once the internal domain is activated. | ||
| + | </span></p></div></html> | ||
| + | |||
| + | ==Characterization of the improved part by AFCM-Egypt 2023 team== | ||
| + | |||
| + | In order to amplify this DNA part, we used PCR amplification to reach the desired concentration to complete our experiments using specific forward and reverse primers, running the parts on gel electrophoresis as this part presents in lane (P2) including STEAP3 and SDC4 and lane (P3) including NAdB, and then measuring the specific concentration of the running part using Real-Time PCR as shown in the following figure. | ||
| + | <html><div align="center"style="border:solid #17252A; width:80%;float:center;"><img style=" max-width:850px; | ||
| + | width:100%; | ||
| + | height:auto; | ||
| + | position: relative; | ||
| + | top: 50%; | ||
| + | left: 50%; | ||
| + | transform: translate( -50%); | ||
| + | padding-bottom:25px; | ||
| + | padding-top:25px; | ||
| + | "src="https://static.igem.wiki/teams/4586/wiki/parts-experiments/pcr-ampli.png"> | ||
| + | <p class=MsoNormal align=center style='text-align:left;border:none;width:98% ;justify-content:center;'><span | ||
| + | lang=EN style='font-size:11.0pt;line-height:115%'> | ||
| + | |||
| + | </span></p></div></html> | ||
| + | <br><br><br><br> | ||
| + | We performed the double digestion method for this part in the prefix and suffix with its specific restriction enzyme and applied this part to gel electrophoresis as shown in the following figure in lane (P2) and (P3). | ||
| + | <html><div align="center"style="border:solid #17252A; width:80%;float:center;"><img style=" max-width:850px; | ||
| + | width:100%; | ||
| + | height:auto; | ||
| + | position: relative; | ||
| + | top: 50%; | ||
| + | left: 50%; | ||
| + | transform: translate( -50%); | ||
| + | padding-bottom:25px; | ||
| + | padding-top:25px; | ||
| + | "src="https://static.igem.wiki/teams/4586/wiki/parts-experiments/digestion-2.png"> | ||
| + | <p class=MsoNormal align=center style='text-align:left;border:none;width:98% ;justify-content:center;'><span | ||
| + | lang=EN style='font-size:11.0pt;line-height:115%'> | ||
| + | |||
| + | </span></p></div></html> | ||
| + | <br><br> | ||
| + | After the ligation step, we did culture of the ligated product to specifically select the optimum colonies to screen it using Colony PCR to make sure that our parts were correctly ligated in the plasmid. | ||
| + | The cell culture plate of transformed pCDNA vector containing insert parts is shown in the following figure. | ||
| + | This plasmid contains | ||
| + | 1-Syn-notch (CD8 alpha-his tag-Anti CD19-mouse notch core-ZF21.16\VP64)) | ||
| + | 2-Booster gene 1 (SDC4, STEAP3) | ||
| + | 3-Booster gene 2 (NAdB) | ||
| + | <br> | ||
| + | <html><div align="center"style="border:solid #17252A; width:75%;float:center;"><img style=" max-width:850px; | ||
| + | width:75%; | ||
| + | height:auto; | ||
| + | position: relative; | ||
| + | top: 50%; | ||
| + | left: 35%; | ||
| + | transform: translate( -50%); | ||
| + | padding-bottom:25px; | ||
| + | padding-top:25px; | ||
| + | "src="https://static.igem.wiki/teams/4586/wiki/parts/vector-1.png"> | ||
| + | <p class=MsoNormal align=center style='text-align:left;border:none;width:98% ;justify-content:center;'><span | ||
| + | lang=EN style='font-size:11.0pt;line-height:115%'> | ||
| + | |||
| + | </span></p></div></html> | ||
===Experimental results=== | ===Experimental results=== | ||
| Line 45: | Line 149: | ||
[[File:2018 LZU-CHINA experimental result1.png|400px|thumb|center|]] | [[File:2018 LZU-CHINA experimental result1.png|400px|thumb|center|]] | ||
<br> | <br> | ||
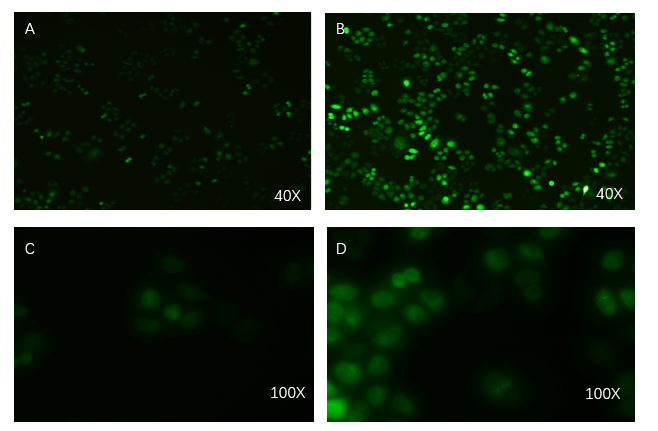
| − | + | PCDH plasmid with exosome booster and <b>CopGFP reporting system</b> was transfected into HEK293T cells stably, and fluorescence before and after transfection was detected by inverted fluorescence microscopy. <b>Figure A is the HEK293T cells of the untransformed plasmid, while figure B is the HEK293T cells of the transformed exosome booster</b>. It can be observed that transfected cells can be stimulated to produce intense fluorescence. Figure. C and D were the fluorescence images of the control group at 100 times (figure. C) and the experimental group (figure. D).</li> | |
<br><li><b>Transmission electron microscopy (TEM) detection</b><br/> | <br><li><b>Transmission electron microscopy (TEM) detection</b><br/> | ||
[[File:2018 LZU-CHINA experimental result2.png|400px|thumb|center|]] | [[File:2018 LZU-CHINA experimental result2.png|400px|thumb|center|]] | ||
<br> | <br> | ||
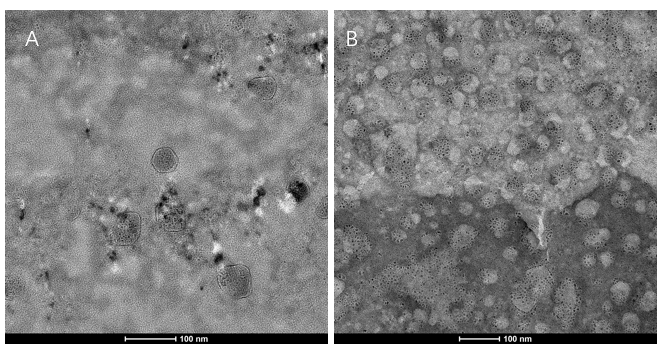
| − | + | We extracted exosomes from HEK293T cell and negatively stained it with phosphotungstic acid. Subsequently, <b>we use TEM to detect it in 100nm</b> . The experimental results demonstrated that HEK293T expressed exosome booster genes (figure B) could dramatically increase its exosome secretion compare with control cell (figure A) </li> | |
<br><li><b>NTA analysis</b><br/> | <br><li><b>NTA analysis</b><br/> | ||
<br> | <br> | ||
[[File:2018 LZU-CHINA experimental result3.png|300px|thumb|center|]] | [[File:2018 LZU-CHINA experimental result3.png|300px|thumb|center|]] | ||
<br> | <br> | ||
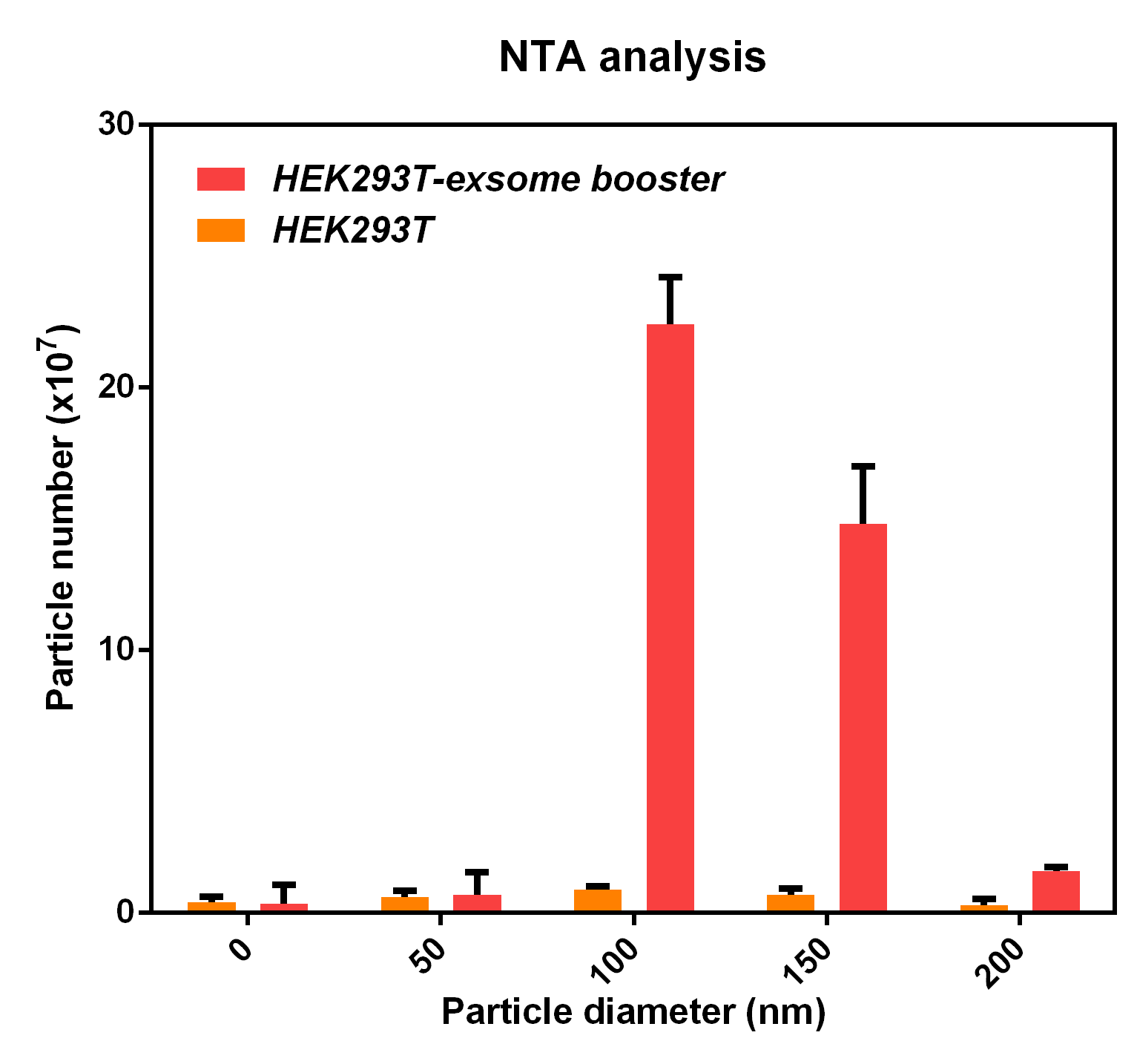
| − | + | The NTA analysis refers to the nanoparticle tracking analysis. <b>The experimental results showed that HEK293T cell overexpressing exosome booster had significantly higher granules numbers at 100nm and 250nm</b> than the control cell (about 23 times as much as the control cell). This indicates that exosome booster can increase the number of exosomes secreted by 293T cell. | |
<br> | <br> | ||
<br><li><b>Exosome uptake experiments</b><br/></li> | <br><li><b>Exosome uptake experiments</b><br/></li> | ||
| Line 60: | Line 164: | ||
[[File:2018 LZU-CHINA experimental result4.png|400px|thumb|center|]] | [[File:2018 LZU-CHINA experimental result4.png|400px|thumb|center|]] | ||
<br> | <br> | ||
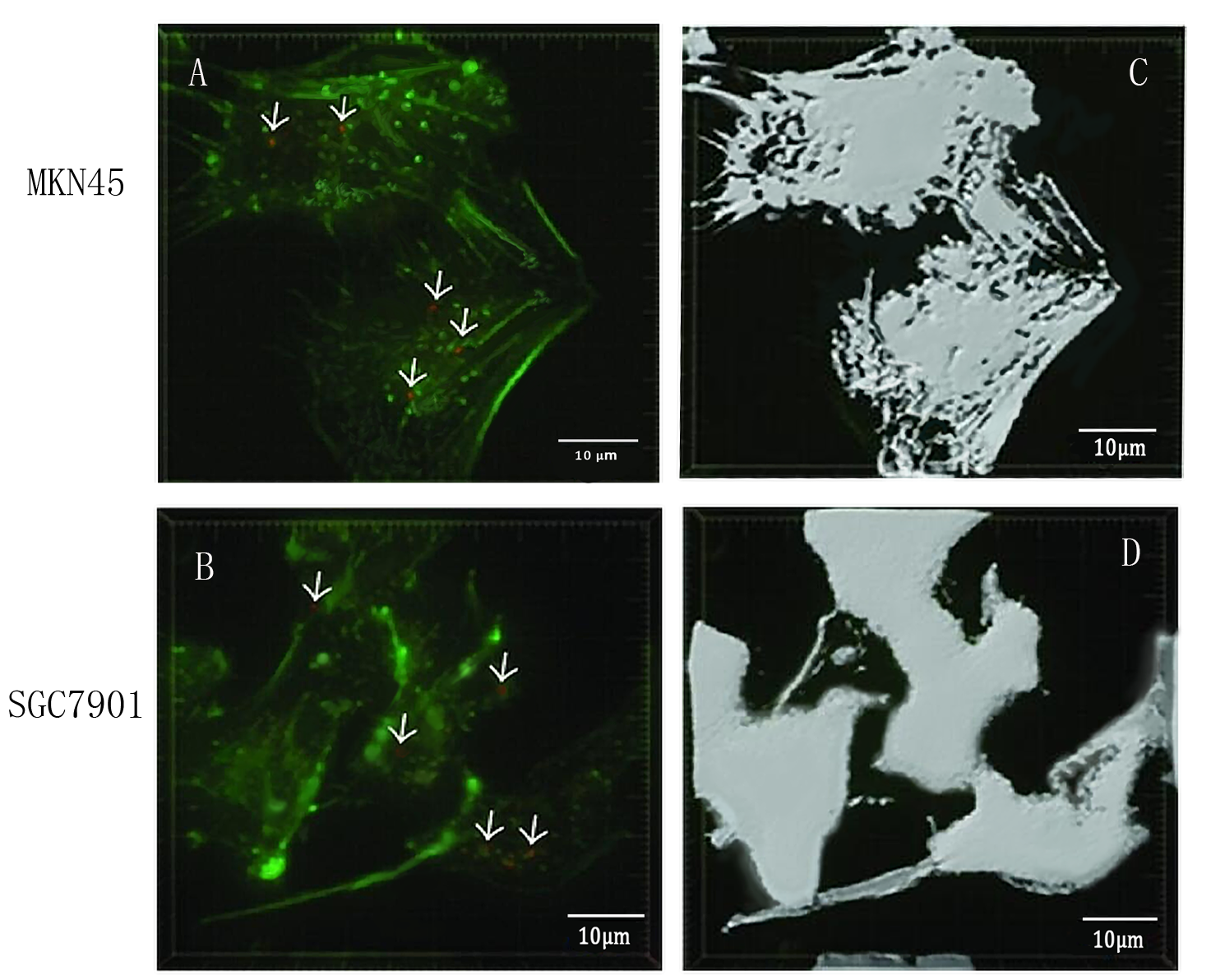
| − | + | To detect whether exosomes whether could enter gastric cancer cells,<b>we used confocal microscopy to observe the uptake of gastric cancer cells MKN45 and SGC7901 with exosomes</b> . First, exosomes were extracted from 293T cells and stained with PKH26 exosomes (red fluorescent dye). The MKN45 and SGC7901 cells were stained with a FITC dye. Exosomes and gastric cancer cells were incubated together for 6 hours. Subsequently, the uptake of exosomes by gastric cancer cells was examined by fluorescence confocal microscopy. Figure. A and C indicate that gastric cancer cells MKN45 and SGC7901 can ingested exosomes. Figure. B and D showed the cytoskeletal structures of MKN45 and SGC7901 in gastric cancer cells detected by β-tubulin antibody. | |
| − | + | ===Reference=== | |
| + | Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson's disease treatment.R Kojima,et.al,2018 | ||
| + | ==Contribution: NJU-China 2020== | ||
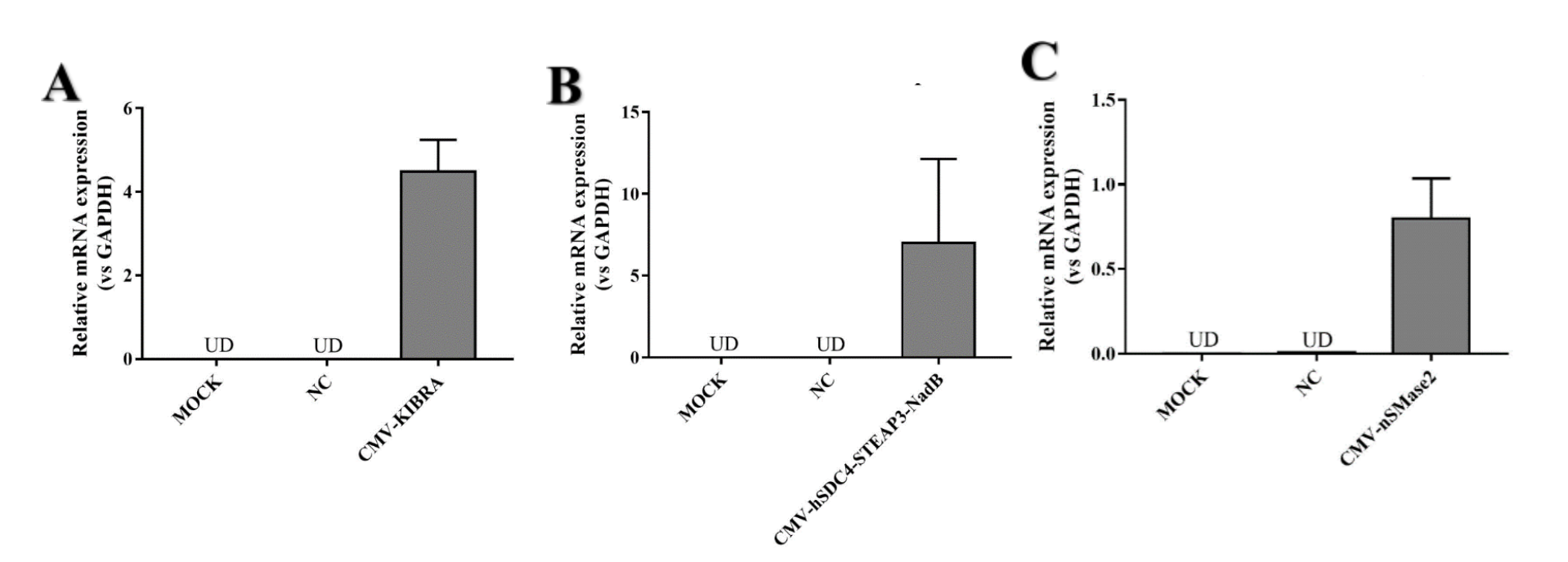
| + | CMV-hSDC4-STEAP3-NadB can strongly promote exosome release. We designed two other parts (CMV-KIBRA and CMV-nSMase2) to promote exosome release. We wanted to find the part that promoted exosome release the most .We detected both mRNA levels. Because CMV-HSDC4-Steap3-NADB has been a very common element to promote exosome release, we only performed RT-qPCR to detect mRNA expression. | ||
| + | [[File:T--NJU-China--13.png|600px|thumb|center|Figure4.(A) KIBRA mRNA relative expression in HEK293T cell (vs GADPH) | ||
| + | <br/>(B) NadB mRNA relative expression in HEK293T cell (vs GADPH)<br/>(C) nSMase2 mRNA relative expression in HEK293T cell (vs GAPDH)]] | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | After confirming the normal expression of the three parts, we attempted to compare the exosomes produced by the three parts. The results showed that KIBRA produced higher exosomes than the other two components. | ||
| + | [[File:T--NJU-China--14.png|300px|thumb|center|Figure5. Total amounts of exosomes (shown as total protein) secreted by HEK293 cells with the introduction of nSMase2, KIBRA and hSDC4-STEAP3-NadB.]] | ||
| + | <br/> | ||
| + | <br/> | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| + | <br><br> | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
Latest revision as of 14:10, 12 October 2023
Exosome booster
- Considering that TIL cells can't secret enough exosomes under normal condition, we fuse three genes to increase the number of exosomes (Alenquer, 2015), thus increasing the transfer efficiency of exosomes.
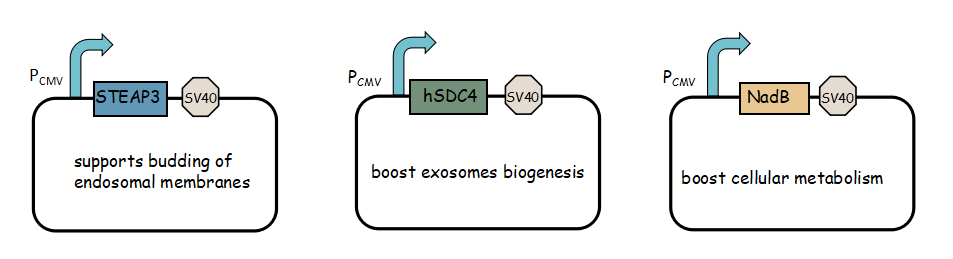
- Exosome booster refers to hSDC4-T2A-STEAP3-P2A-NadB.We identified STEAP3 (involved in exosomebiogenesis), syndecan-4(SDC4; supports budding of endosomal membranes to form multivesicular bodies), and a fragment of L-aspartate oxidase (NadB; possibly boosts cellular metabolism by tuning up the citric acid cycle) as potential synthetic exosome production boosters. Combined expression of these genes significantly increased exosome production.(Ryosuke Kojima et al. 2008)
-
They combine with each other to boost exosomal transfer efficiency:
-
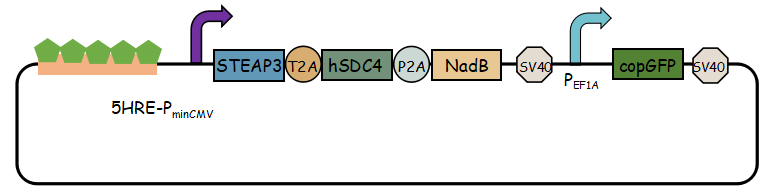
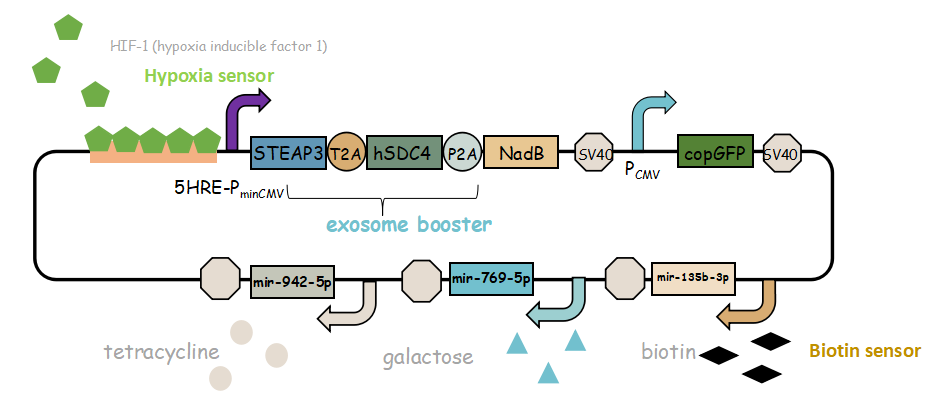
In previous article, it use IRES (internal ribosome entry site,IRES) to connect three genes, we change it into linkers T2A and P2A to ensure proper expression of three genes.Then we use use reporter gene copGFP to manifest the expression of exosome booster with the promoter of 5-HRE-PminCMV.
- Here is the final goal that we designed for this project.
-
It combines with exosome booster and miR attacker (miR135b-3p, miR-942-5p,miR768-5p) under control of induced promoter (hypoxia,tetracycline, biotin and galactose induced system). By adding different inducers into the cell culture, we can prompt engineered 293T cells to express different concentrations of miRNA. There are six combinations; then we can find the optimum combination of miRNA to achieve our goal——kill tumor cells.
More design details and description you can obtain from our wiki!
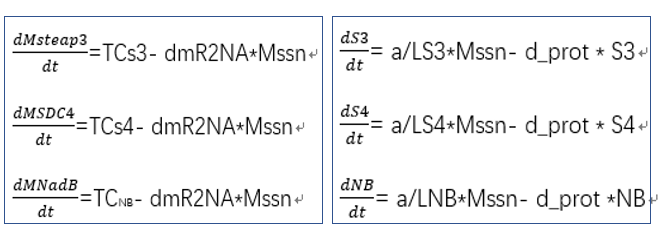
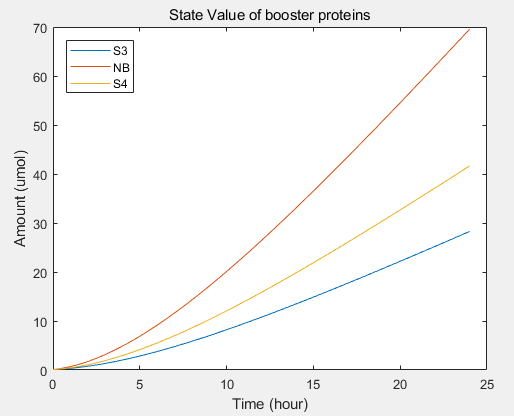
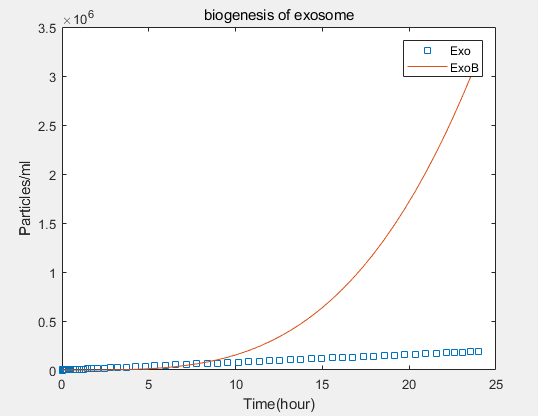
- transcriptional rate simulation of three booster genes(left) and simulation of three booster genes transcriptional translation(right):
From the formula, we can obtain three model about exosome booster.
a.the promotional concentrations of the three promotional genes for the secretion of exosomes:Over time, the number of exosomes secreted by cells increased.
b.Comparison between the expression levels of three promoter genes and those of non-promoter exosomes:It demonstrates that exosome booster could dramatically increase exosome secrion.
c.production rate of exosomes in cell:This model predicts the production rate of exosome over time in cells.
Improvement by AFCM-Egypt 2023 team
This composite part is an improvement for (BBa_K2796028) Designed by (iGEM18_LZU-CHINA).We developed this new approach to control the expression of these booster genes (SDC4, STEAP3, NadB) that enhance the exosomes secretion within our engineered mesenchymal stem cell, as we found out that constitutive overexpression of these genes may carry out multiple side effect in addition to depleting MSCs resources and impairing its function and survival, so we implemented the Cre loxP system for conditional gene expression to induce the expression of these booster genes by deleting (STOP) sequence that prevents the transcription of the downstream genes. Thus, the expression of booster genes as condition in the presence of Cre recombinase enzyme after vimentin syn notch activation.
Presence of SDC4 as a booster gene has a role in increasing the concentration of the produced engineered exosomes so it plays an effective role to increase the efficacy of the therapeutic agent.
This figure illustrates the conditional expression of our enhanced booster genes that increase the exosomal secretion.
We compared both condition of exosomes production when using booster genes and without it
(1)No booster genes with conditioned release
This Represents the relation between the activation of the internal domain of the syn-noth (represented as red line) and production of exosomes with specific cargo (represented as blue line) as the production of the engineered exosomes is initiated once the internal domain is activated.
(2)Booster gene with conditioned release
This Represents the relation between the activation of the internal domain of the syn-noth (represented as red line) and production of exosomes with specific cargo (represented as blue line) as the production of the engineered exosomes is initiated once the internal domain is activated.
Characterization of the improved part by AFCM-Egypt 2023 team
In order to amplify this DNA part, we used PCR amplification to reach the desired concentration to complete our experiments using specific forward and reverse primers, running the parts on gel electrophoresis as this part presents in lane (P2) including STEAP3 and SDC4 and lane (P3) including NAdB, and then measuring the specific concentration of the running part using Real-Time PCR as shown in the following figure.

We performed the double digestion method for this part in the prefix and suffix with its specific restriction enzyme and applied this part to gel electrophoresis as shown in the following figure in lane (P2) and (P3).
After the ligation step, we did culture of the ligated product to specifically select the optimum colonies to screen it using Colony PCR to make sure that our parts were correctly ligated in the plasmid. The cell culture plate of transformed pCDNA vector containing insert parts is shown in the following figure. This plasmid contains 1-Syn-notch (CD8 alpha-his tag-Anti CD19-mouse notch core-ZF21.16\VP64)) 2-Booster gene 1 (SDC4, STEAP3) 3-Booster gene 2 (NAdB)

Experimental results
- Exosome booster can enhance the ability of cells to secrete exosomes and it was expressed in HEK293T by lentiviral vector to verify its function. The experiments includes three parts: the transfection effect of exosome booster was detected by an inverted fluorescence microscope. The presence of exosome was detected by electron microscopy and the effect of exosome booster on the exosome secretion of HEK293T was detected by NTA analysis.
- Exosome-booster stable cell line construction
PCDH plasmid with exosome booster and CopGFP reporting system was transfected into HEK293T cells stably, and fluorescence before and after transfection was detected by inverted fluorescence microscopy. Figure A is the HEK293T cells of the untransformed plasmid, while figure B is the HEK293T cells of the transformed exosome booster. It can be observed that transfected cells can be stimulated to produce intense fluorescence. Figure. C and D were the fluorescence images of the control group at 100 times (figure. C) and the experimental group (figure. D).
- Transmission electron microscopy (TEM) detection
We extracted exosomes from HEK293T cell and negatively stained it with phosphotungstic acid. Subsequently, we use TEM to detect it in 100nm . The experimental results demonstrated that HEK293T expressed exosome booster genes (figure B) could dramatically increase its exosome secretion compare with control cell (figure A)
- NTA analysis
The NTA analysis refers to the nanoparticle tracking analysis. The experimental results showed that HEK293T cell overexpressing exosome booster had significantly higher granules numbers at 100nm and 250nm than the control cell (about 23 times as much as the control cell). This indicates that exosome booster can increase the number of exosomes secreted by 293T cell.
- Exosome uptake experiments
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 192
Illegal BglII site found at 1777 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 2657
Illegal AgeI site found at 3277 - 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI site found at 1367
Modeling
To increase the expression of exosomes, we added three enhanced genes to simulate the effect of increasing exosomes secretion through the model, providing guidance for our experiment. It includes three equations:
To detect whether exosomes whether could enter gastric cancer cells,we used confocal microscopy to observe the uptake of gastric cancer cells MKN45 and SGC7901 with exosomes . First, exosomes were extracted from 293T cells and stained with PKH26 exosomes (red fluorescent dye). The MKN45 and SGC7901 cells were stained with a FITC dye. Exosomes and gastric cancer cells were incubated together for 6 hours. Subsequently, the uptake of exosomes by gastric cancer cells was examined by fluorescence confocal microscopy. Figure. A and C indicate that gastric cancer cells MKN45 and SGC7901 can ingested exosomes. Figure. B and D showed the cytoskeletal structures of MKN45 and SGC7901 in gastric cancer cells detected by β-tubulin antibody.
Reference
Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson's disease treatment.R Kojima,et.al,2018
Contribution: NJU-China 2020
CMV-hSDC4-STEAP3-NadB can strongly promote exosome release. We designed two other parts (CMV-KIBRA and CMV-nSMase2) to promote exosome release. We wanted to find the part that promoted exosome release the most .We detected both mRNA levels. Because CMV-HSDC4-Steap3-NADB has been a very common element to promote exosome release, we only performed RT-qPCR to detect mRNA expression.
After confirming the normal expression of the three parts, we attempted to compare the exosomes produced by the three parts. The results showed that KIBRA produced higher exosomes than the other two components.
Sequence and Features