Difference between revisions of "Part:BBa K2596001"
m (→Usage and Biology) |
(→Activity of synthetic variants of cpcB promoter) |
||
| (28 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K2596001 short</partinfo> | <partinfo>BBa_K2596001 short</partinfo> | ||
| − | PcpcB is a constitutive promoter from cyanobacteria Synechococcus elongatus PCC 7942. | + | <em>PcpcB</em> is a constitutive promoter from cyanobacteria <i>Synechococcus elongatus</i> PCC 7942 [1]. |
<!-- Add more about the biology of this part here --> | <!-- Add more about the biology of this part here --> | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| − | cpcB codes for phycocyanin, a phycobiliprotein complex, and works to harvest light energy before transmitting it to the chlorophylls. The promoter is a strong promoter but also demonstrates expression patterns following circadian oscillations. Studies have found that Pcpc can also be used in E. coli. | + | <i>cpcB</i> codes for phycocyanin, a phycobiliprotein complex, and works to harvest light energy before transmitting it to the chlorophylls. The promoter is a strong promoter but also demonstrates expression patterns following circadian oscillations. Studies have found that <i>Pcpc</i> can also be used in <em>E. coli</em>. |
<!-- --> | <!-- --> | ||
| Line 14: | Line 14: | ||
<partinfo>BBa_K2596001 SequenceAndFeatures</partinfo> | <partinfo>BBa_K2596001 SequenceAndFeatures</partinfo> | ||
| − | PcpcB1A1 features two promoter sequences within the promoter, both of which were included in the BioBrick. For this promoter, derived from Synechococcus elongatus PCC 7942, we kept about 100 bp of upstream and downstream regions, the latter of which was cropped immediately before the ATG start codon. | + | <i>PcpcB1A1</i> features two promoter sequences within the promoter, both of which were included in the BioBrick. For this promoter, derived from <i>Synechococcus elongatus</i> PCC 7942, we kept about 100 bp of upstream and downstream regions, the latter of which was cropped immediately before the ATG start codon. |
| − | + | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
| Line 21: | Line 20: | ||
<partinfo>BBa_K2596001 parameters</partinfo> | <partinfo>BBa_K2596001 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | |||
| + | ===Luciferase Assays=== | ||
| + | |||
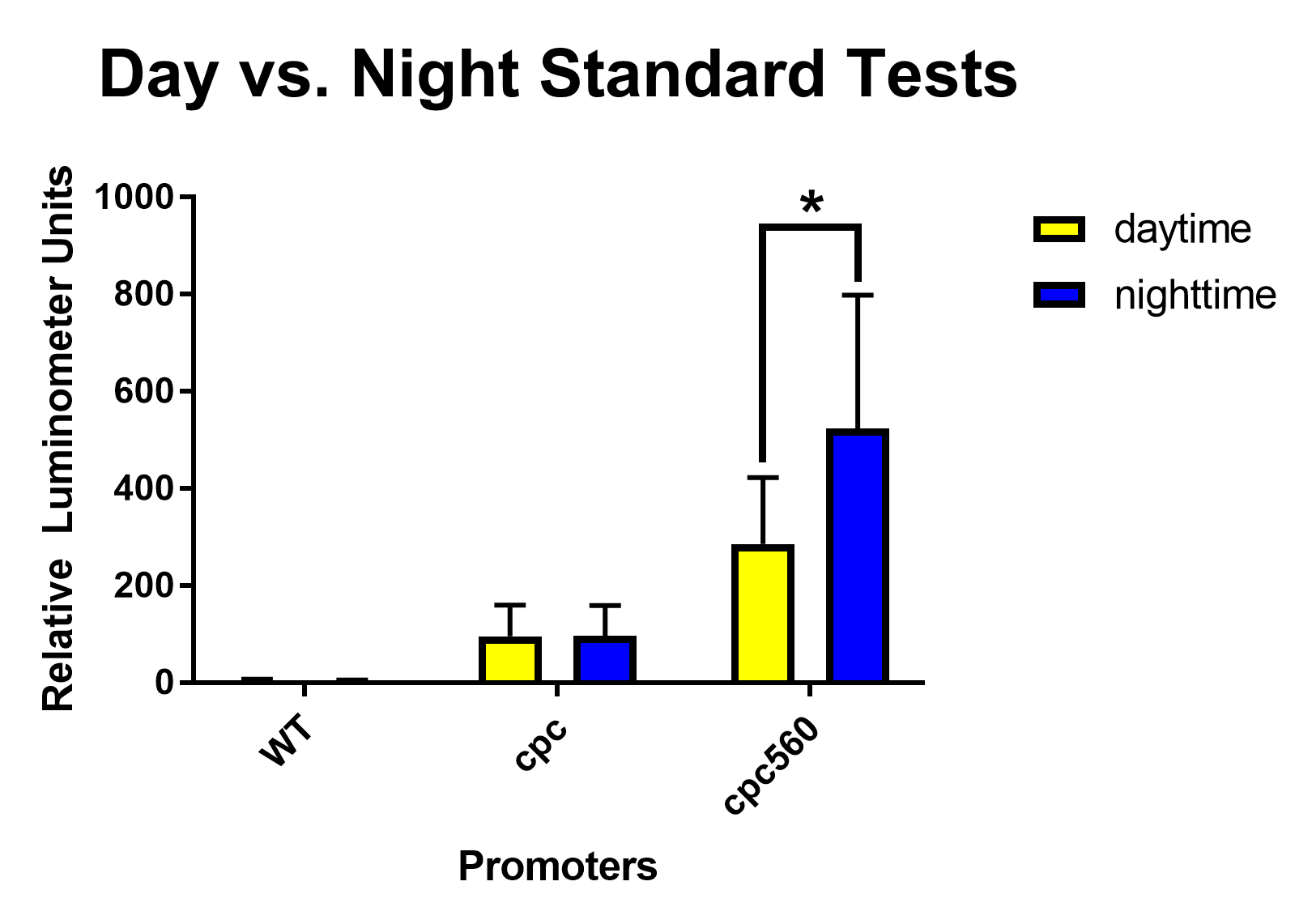
| + | [[File: T--Stony Brook--Results day vs night.png|thumb|Figure 1. Daytime and nighttime standard expression tests for the constitutive promoters cpc and cpc-560.]] | ||
| + | |||
| + | [[File:T--Stony Brook daytime 2.png|thumb|Figure 2. Daytime Standard Expression of luxAB for the constitutive promoters cpc and cpc-560, ferrous ion repressible idiA, and high light inducible psbA2 promoters]] | ||
| + | |||
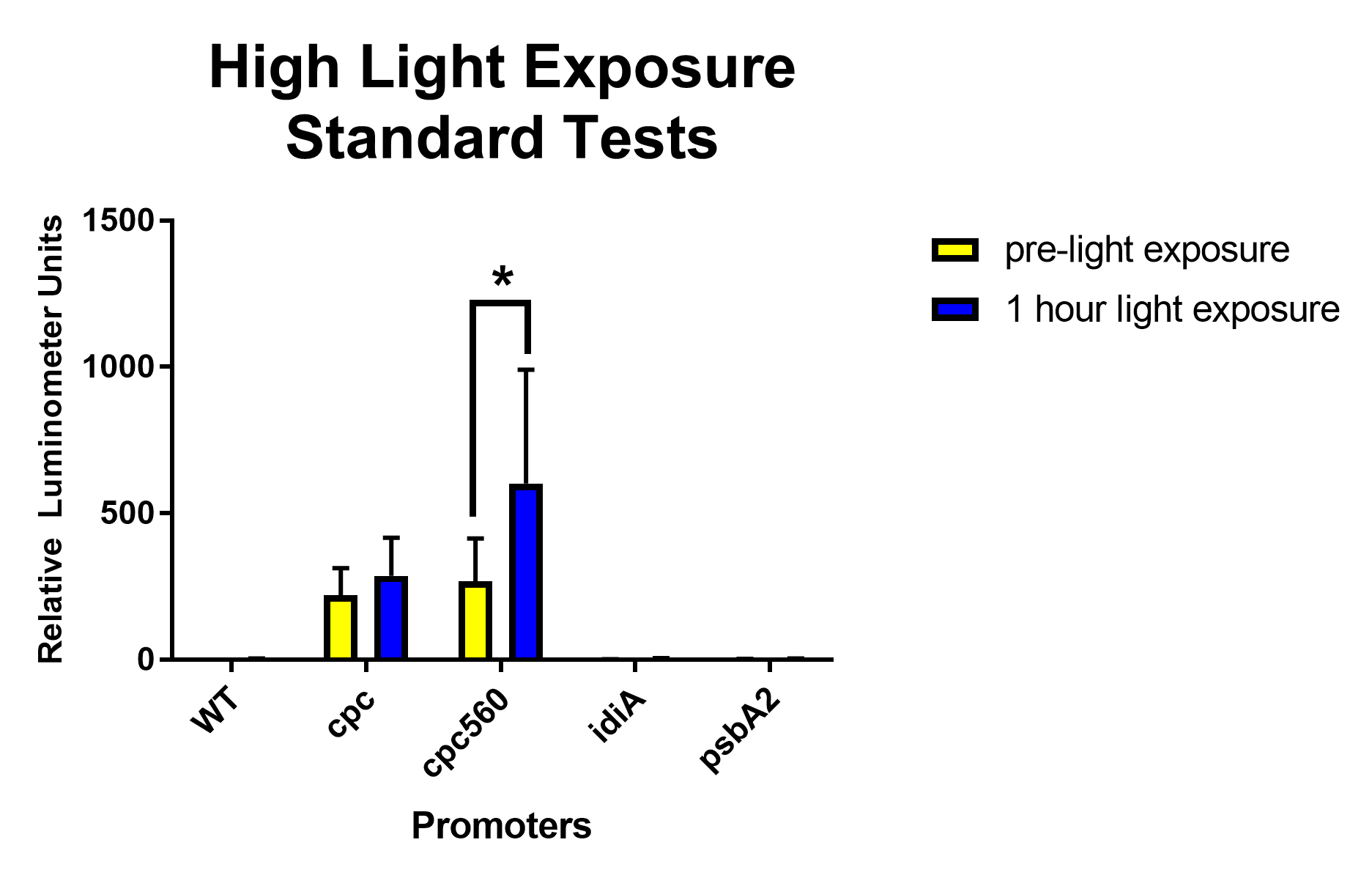
| + | [[File: T--Stony Brook--Results high light exposure.png|thumb|Figure 3. High light exposure Standard Expression tests for the constitutive promoters cpc and cpc-560, ferrous ion repressible idiA, and high light inducible psbA2 promoters]] | ||
| + | |||
| + | [[File: T--Stony Brook--Results Effects of Iron Chelator.png|thumb|Figure 4. Effect of Iron Chelator on expression for the constitutive promoters cpc and cpc-560, ferrous ion repressible idiA, and high light inducible psbA2 promoters]] | ||
| + | |||
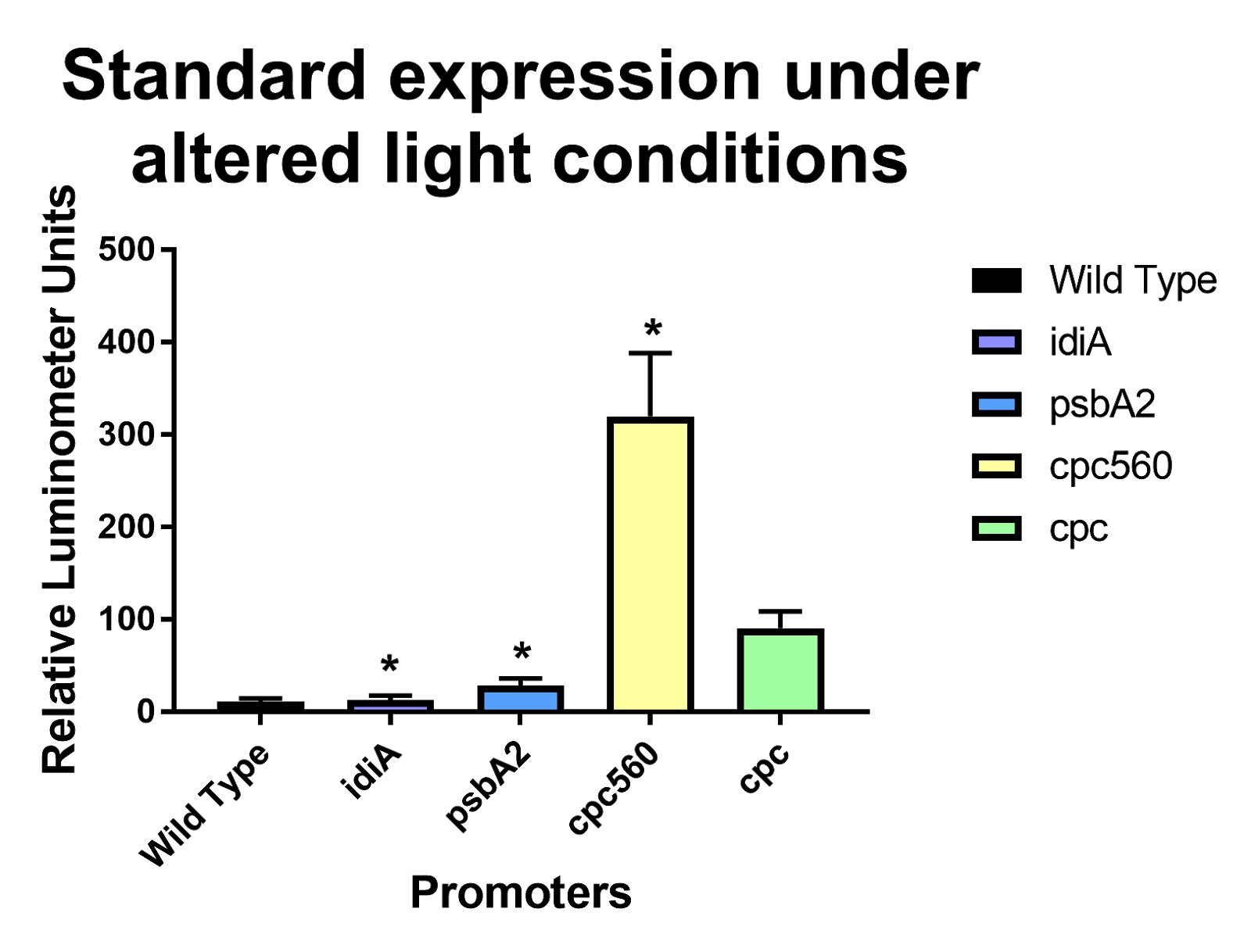
| + | [[File: T--Stony Brook--Results alternative light.png|thumb|Figure 5. Standard Expression tests under altered light conditions for the constitutive promoters cpc and cpc-560, ferrous ion repressible idiA, and high light inducible psbA2 promoters]] | ||
| + | |||
| + | Experimental Methods: | ||
| + | |||
| + | In order to test the expression of our promoters, <i>cpc</i> (BBa_K2596001), <i>cpc560</i> (BBa_K2596006), <i>idiA</i> (BBa_K2596004), and <i>psbA2</i> (BBa_K2596003), which were incorporated into Dr. Susan Golden’s vector pAM1414 using Gibson assembly, we conducted luciferase experiments. Following Dr. Golden’s procedure, we added 5 µL of decanal to 95 µL of cyanobacteria in each well to induce expression. The decanal acted as a substrate for the bacterial luciferase enzyme, but due to the toxicity, the cells ended up dying, so the data obtained represents the end static expression. For the standard expression experiments for nighttime and daytime, we plated 95 µL of cyanobacteria into a 96 well plate, added 5 µL of decanal, parafilmed the edges and left the plate for 15 minutes before measuring the luminescence in a plate reader. For the high light experiment to <i>psbA2</i> promoter, we plated 95 µL of cyanobacteria to half of the wells and put them under at least 500 µE of high light in our incubator for 1 hour. Then, we added 95 µL of cyanobacteria not exposed to high light to the other half of the wells and added 5 µL of decanal to all of the wells. Then, we placed the well plate in a plate reader and measured the luminescence. For the iron repressible experiment to <i>idiA</i> promoter, we plated 95 µL of cyanobacteria to half of the wells and exposed them to 2.3 mM of iron chelating agent 2,2-dipyridyl for one hour. Then, we added 95 µL of cyanobacteria not exposed to the iron chelating agent to the other half of the wells and added 5 µL of decanal to all of the wells. We placed the well plate in a plate reader and measured the luminescence. | ||
| + | |||
| + | Results: | ||
| + | |||
| + | After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, both <i>cpc</i> and <i>cpc-560</i> showed significant differences for daytime expression compared to wild type [Figure 1]. Compared to <i>cpc</i>, <i>cpc-560</i> had a significantly higher expression [Figure 1]. In comparison for day compared to night, <i>cpc</i> did not show any significant difference [Figure 2]. On the other hand, <i>cpc-560</i> showed a significant difference between daytime and nighttime expression [Figure 2]. | ||
| + | After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, for daytime expression, compared to all other promoters and the wild type, <i>cpc</i> and <i>cpc-560</i> had significant differences in expression [Figure 1]. For <i>idiA</i> and <i>psbA2</i>, they only had significant differences compared to cpc and cpc-560, and not to each other [Figure 1]. When exposed to highlight, <i>idiA</i> and <i>psbA2</i> did not show any expression [Figure 3]. | ||
| + | |||
| + | After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, for expression after iron repression, <i>idiA</i>, <i>psbA2</i>, and <i>cpc-560</i> all showed a significant difference between before iron chelator reagent addition and after [Figure 4]. <i>cpc</i>’s insignificant difference could be attributed to the fact that there were only two cpc samples, as a result of insufficient cyanobacterial growth. The significant decrease in the expression of <i>cpc-560</i>, however, as a result of adding the iron chelator, indicates that the cells may have died from prolonged deficiency of ferrous ions, therefore that one hour may have been too long [Figure 4]. | ||
| + | |||
| + | When conducting our ferrous standard expression experiments, we had left the untreated cyanobacteria as well as the treated cyanobacteria in our biosafety cabinet, not expecting any major changes in expression due to the light exposure. However, under these light conditions, <i>psbA2</i> was expressed significantly more than wild type [Figure 5]. We propose that this may be due to our incubators having excess light intensity or red light, which may have inhibited <i>psbA2</i> expression. | ||
| + | |||
| + | |||
| + | ===Activity of synthetic variants of ''cpcB'' promoter === | ||
| + | <strong>IISER-Pune-India 2021:</strong><be> | ||
| + | |||
| + | The ''cpcB'' promoter has been found to be repressed under high light intensities [1]. Sengupta ''et al.'' (2018) created synthetic mutant versions of the ''cpcB'' promoter through error prone PCR with the aim of generating variants of ''cpcB'' that show constitutive expression even under high light conditions. ''cpcB m6'' and ''cpcB m9'' are two such mutants that showed increased expression in Synechococcus elongatus PCC 7942 at a high light condition of 300 umol photons/m2/s compared to the low light condition of 100 umol photons/m2/s. They also showed statistically significantly higher expression at high light conditions compared to the wild type ''cpcB'' promoter. This characterisation was also conducted in ''S. elongatus'' PCC 11801 and PCC 11802. | ||
| + | |||
| + | The promoter variants were characterised at 1% CO2 concentrations via the fluorescence produced by an eYFP reporter. Standardisation was done by converting the relative fluorescence values to milligrams of protein/ gram DCW using the standard curve of the purified eYFP and the dry cell weight (DCW) of the respective organisms. | ||
| + | |||
| + | ''cpcB m6'' differs from the wild type promoter in terms of one base pair substitution at the 24th nucleotide: G is substituted by A. ''cpcB m9'' has one deletion at the 22nd nucleotide (a thymine), relative to the wild type. | ||
| + | |||
| + | |||
| + | The registry page for ''cpcB m6'' can be found at [[Part:BBa_K3971000]]. | ||
| + | |||
| + | [[File:T--IISER-Pune-India--sengupta.png|thumb|700x770px|centre|Fig. 1: Characterisation of mutants of the ''cpcB'' promoter in ''S. elongatus'' PCC 7942, PCC 11801 and PCC 11802. Mutant versions were characterised in the three chasses via an eYFP reporter gene at 1% CO<sub>2</sub> concentrations and high light conditions - 300 umol photons/m2/s (red) or low light conditions - 100 umol photons/m2/s (black). Relative fluorescence of reporter was converted to milligrams of protein/gram dry cell weight. * indicates statistically significant difference between mutant promoter and wild type ''cpcB'' promoter (p<0.05). ''Figure adapted from Sengupta et al. (2020)]] | ||
| + | |||
| + | ===References:=== | ||
| + | |||
| + | [1] Sengupta, Annesha, et al. "Fine-tuning native promoters of Synechococcus elongatus PCC 7942 to develop a synthetic toolbox for heterologous protein expression." ACS synthetic biology 8.5 (2019): 1219-1223. | ||
Latest revision as of 20:26, 20 October 2021
PcpcB from Synechococcus elongatus PCC7942
PcpcB is a constitutive promoter from cyanobacteria Synechococcus elongatus PCC 7942 [1].
Usage and Biology
cpcB codes for phycocyanin, a phycobiliprotein complex, and works to harvest light energy before transmitting it to the chlorophylls. The promoter is a strong promoter but also demonstrates expression patterns following circadian oscillations. Studies have found that Pcpc can also be used in E. coli.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
PcpcB1A1 features two promoter sequences within the promoter, both of which were included in the BioBrick. For this promoter, derived from Synechococcus elongatus PCC 7942, we kept about 100 bp of upstream and downstream regions, the latter of which was cropped immediately before the ATG start codon.
Luciferase Assays
Experimental Methods:
In order to test the expression of our promoters, cpc (BBa_K2596001), cpc560 (BBa_K2596006), idiA (BBa_K2596004), and psbA2 (BBa_K2596003), which were incorporated into Dr. Susan Golden’s vector pAM1414 using Gibson assembly, we conducted luciferase experiments. Following Dr. Golden’s procedure, we added 5 µL of decanal to 95 µL of cyanobacteria in each well to induce expression. The decanal acted as a substrate for the bacterial luciferase enzyme, but due to the toxicity, the cells ended up dying, so the data obtained represents the end static expression. For the standard expression experiments for nighttime and daytime, we plated 95 µL of cyanobacteria into a 96 well plate, added 5 µL of decanal, parafilmed the edges and left the plate for 15 minutes before measuring the luminescence in a plate reader. For the high light experiment to psbA2 promoter, we plated 95 µL of cyanobacteria to half of the wells and put them under at least 500 µE of high light in our incubator for 1 hour. Then, we added 95 µL of cyanobacteria not exposed to high light to the other half of the wells and added 5 µL of decanal to all of the wells. Then, we placed the well plate in a plate reader and measured the luminescence. For the iron repressible experiment to idiA promoter, we plated 95 µL of cyanobacteria to half of the wells and exposed them to 2.3 mM of iron chelating agent 2,2-dipyridyl for one hour. Then, we added 95 µL of cyanobacteria not exposed to the iron chelating agent to the other half of the wells and added 5 µL of decanal to all of the wells. We placed the well plate in a plate reader and measured the luminescence.
Results:
After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, both cpc and cpc-560 showed significant differences for daytime expression compared to wild type [Figure 1]. Compared to cpc, cpc-560 had a significantly higher expression [Figure 1]. In comparison for day compared to night, cpc did not show any significant difference [Figure 2]. On the other hand, cpc-560 showed a significant difference between daytime and nighttime expression [Figure 2]. After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, for daytime expression, compared to all other promoters and the wild type, cpc and cpc-560 had significant differences in expression [Figure 1]. For idiA and psbA2, they only had significant differences compared to cpc and cpc-560, and not to each other [Figure 1]. When exposed to highlight, idiA and psbA2 did not show any expression [Figure 3].
After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, for expression after iron repression, idiA, psbA2, and cpc-560 all showed a significant difference between before iron chelator reagent addition and after [Figure 4]. cpc’s insignificant difference could be attributed to the fact that there were only two cpc samples, as a result of insufficient cyanobacterial growth. The significant decrease in the expression of cpc-560, however, as a result of adding the iron chelator, indicates that the cells may have died from prolonged deficiency of ferrous ions, therefore that one hour may have been too long [Figure 4].
When conducting our ferrous standard expression experiments, we had left the untreated cyanobacteria as well as the treated cyanobacteria in our biosafety cabinet, not expecting any major changes in expression due to the light exposure. However, under these light conditions, psbA2 was expressed significantly more than wild type [Figure 5]. We propose that this may be due to our incubators having excess light intensity or red light, which may have inhibited psbA2 expression.
Activity of synthetic variants of cpcB promoter
IISER-Pune-India 2021:<be>
The cpcB promoter has been found to be repressed under high light intensities [1]. Sengupta et al. (2018) created synthetic mutant versions of the cpcB promoter through error prone PCR with the aim of generating variants of cpcB that show constitutive expression even under high light conditions. cpcB m6 and cpcB m9 are two such mutants that showed increased expression in Synechococcus elongatus PCC 7942 at a high light condition of 300 umol photons/m2/s compared to the low light condition of 100 umol photons/m2/s. They also showed statistically significantly higher expression at high light conditions compared to the wild type cpcB promoter. This characterisation was also conducted in S. elongatus PCC 11801 and PCC 11802.
The promoter variants were characterised at 1% CO2 concentrations via the fluorescence produced by an eYFP reporter. Standardisation was done by converting the relative fluorescence values to milligrams of protein/ gram DCW using the standard curve of the purified eYFP and the dry cell weight (DCW) of the respective organisms.
cpcB m6 differs from the wild type promoter in terms of one base pair substitution at the 24th nucleotide: G is substituted by A. cpcB m9 has one deletion at the 22nd nucleotide (a thymine), relative to the wild type.
The registry page for cpcB m6 can be found at Part:BBa_K3971000.

References:
[1] Sengupta, Annesha, et al. "Fine-tuning native promoters of Synechococcus elongatus PCC 7942 to develop a synthetic toolbox for heterologous protein expression." ACS synthetic biology 8.5 (2019): 1219-1223.