Difference between revisions of "Part:BBa K2637003"
| (3 intermediate revisions by one other user not shown) | |
(No difference)
| |
Latest revision as of 12:48, 16 October 2018
KaiC Protein of Circadian Rhythm
KaiC is the core of our oscillating system. Phosphorylation and dephosphorylation of KaiC are related to which proteins bind to it. Some of these proteins, for example, SasA(BBa_K2637004) are related to the regulation of downstream genes and then directly affect the biological activities of organisms. We have optimized it in yeast.
Our basic parts range from BBa_K2637001 to BBa_K2637018 and BBa_K2637053 to BBa_K2637059. Our composite parts range from BBa_K2637021 to BBa_K2637044 and BBa_K2637047 to BBa_K2637052. Our part collection include all our basic parts except for BBa_K2637018 and all composite parts.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 262
Illegal BglII site found at 769 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Biology and Usage
Overview of Cyanobacterias' circadian rhythm
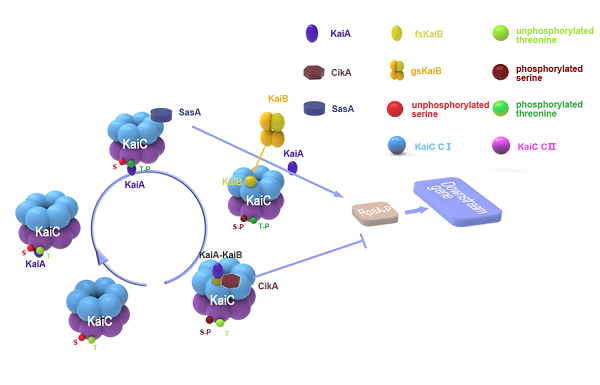
Organisms are adapted to the relentless cycles of day and night, because they evolved timekeeping systems called circadian clocks, which regulate biological activities with ~24-hour rhythms. The clock of cyanobacteria is driven by a three-protein oscillator composed of only three protein KaiA, KaiB and KaiC, which together generate a circadian rhythm of KaiC phosphorylation at residues serine 431 and threonine 432 in the CII dimain. KaiA promotes KaiC (auto)phosphorylation during the subjective day, whereas KaiB provides inhibition of KaiA and promotes KaiC (auto)dephosphorylation during the subjective night. The 24-h KaiC phosphorylation pattern can be reconstituted in vitro by merely combining the three Kai proteins and ATP, suggesting that It is post-transcriptional oscillations and is only related to proteins. Like KaiA, KaiB is also involved in regulating two antagonistic clock-output proteins--SasA and CikA, which reciprocally control the master regulator of transcription RpaA.
Usage of Life Tik Tok (Cyanobacterias' circadian rhythm in yeast)
Life Tik Tok is a circadian rhythm that is established in yeast by iGEM Tianjin, 2018. It enables to regular the biological activities in yeast under the control of proteins in KaiABC oscillator. Learn more about Life Tik Tok and click here.[http://2018.igem.org/Team:Tianjin]
Intereaction between the proteins
Stepwise binding of two KaiA dimers triggers KaiC autophosphorylation at Thr432 and Ser431 . These phosphorylation events enable cooperative binding of fold-switched KaiB monomers to the KaiC-CI domain, forming the KaiCB complex. KaiCB provides a scaffold for the successive sequestration of KaiA in ternary KaiCBA assemblies, concurring with a rearrangement of the KaiA PsR domains. KaiA sequestration promotes KaiC autodephosphorylation, resulting in the regeneration of free KaiC through release of KaiBA subcomplexes. Temporal information from the oscillator is transmitted to downstream genes via the histidine protein kinase SasA (Synechococcus adaptive sensor A), whose autophosphorylation is stimulated by interaction with KaiC. Phosphorylated SasA in turn transfers a phosphoryl group to RpaA (regulator of phycobilisome association A) , a transcription factor that directly regulates the expression of approximately 100 genes. Moreover, RpaA indirectly regulates the expression of nearly all genes in the genome. Disruption of sasA also results in severely damped gene expression rhythms. Surprisingly, the phosphorylation state of RpaA, and subsequently its activity, have been shown to be dependent on CikA, which was primarily thought to be involved in entrainment.
Reconstruction of Life Tik Tok in yeast
When we reconstruct the KaiABC oscillator in yeast, our goals are:
·Characterize the combination between KaiABC changing over time via yeast two-hybrid system
·Explore the role of other proteins in stabilizing the oscillation without involving TTFL
·Discuss the effect of KaiC concentration on oscillation and select the proper promoters
·Research how heterologous circadian clock influences chromosome structure in yeast
We aim to integrate the KaiABC system into Saccharomyces cerevisiae BY4741, and have it control circadian rhythm in yeast. To do this, we have designed three plasmids which can be transformed into yeast to produce a oscillation system and characterized the protein interactions by yeast two-hybrid system. You can learn something about yeast two-hybrid system by clicking here. The first plasmid expresses KaiA, KaiB and KaiC which is fused to a Gal4 activation domain. The second plasmid is our reporter plasmid, which has fluorescent protein promoted by mutant Gal1p and luciferase promoted by Gal2p. The third plasmid contains CikA, SasA and RpaA. We respectively link a Gal4 binding domain with CikA or SasA to find the suitable binding protein that can characterize the oscillation. To explore more protein interactions, KaiB and KaiC were also used as proteins in the yeast two-hybrid system to construct a new oscillation system.These systems would conclusively show that the KaiABC system can work normally in yeast, which serves as a proof of concept for placing eukaryotic gene expression under the control of an exogenous circadian clock. In addition, to make sure that Gal1p and Gal2p can only be activited after the combination of proteins, we deleted two genes in wild-type BY4741. They are Gal4 and Gal80, which can activate or repress Gal1p and Gal2p, respectively. The principle is explained in yeast two-hybrid. And then, we deleted gene BarI to solve the problem of desynchronization between different generations of yeast.
Moreover, we detects the strength of several common promoters in yeast to find the proper one that provides suitable concentration. Thanks to our modeling, we can calculate the range of concentration of KaiC to support the oscillation, which contributes a lot to our selection of promoters.
Characterization of Life Tik Tok
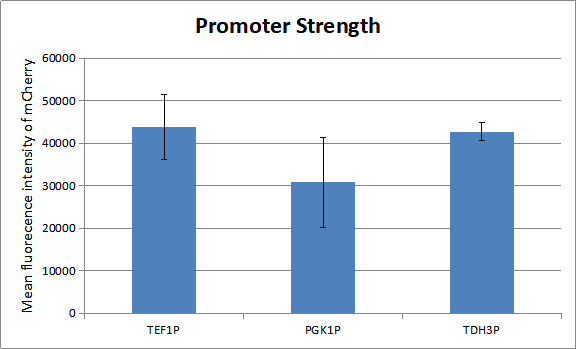
Characterization of promoters
Before our test the whole system, we measured the strength of promoters which regulate the experssion of genes in our oscillator. As there is a natural ratio of the three proteins-KaiA, KaiB and KaiC in cyanobacteria. And a certain ratio of the three proteins has advantage of modeling.
We can draw a conclution about the strength of the three promoters. So we combine TEF1 promoter with KaiA and TEF1 terminator, PGK1 promoter with KaiB and PGK1 terminator and TDH3 promoter with AD-KaiC and ADH1 terminator. Similarly, we combina TEF1 promoter with CikA and TEF1 terminator, PGK1 promoter with RpaA and PGK1 terminator and TDH3 promoter with BD-SasA and ADH1 terminator. And here is only one combination of our experiment. To get more combinations, please see the part from BBa_K2637027 to BBa_K2637038.You can see more about our project to click here.[http://2018.igem.org/Team:Tianjin]
Characterization of Life Tik Tok
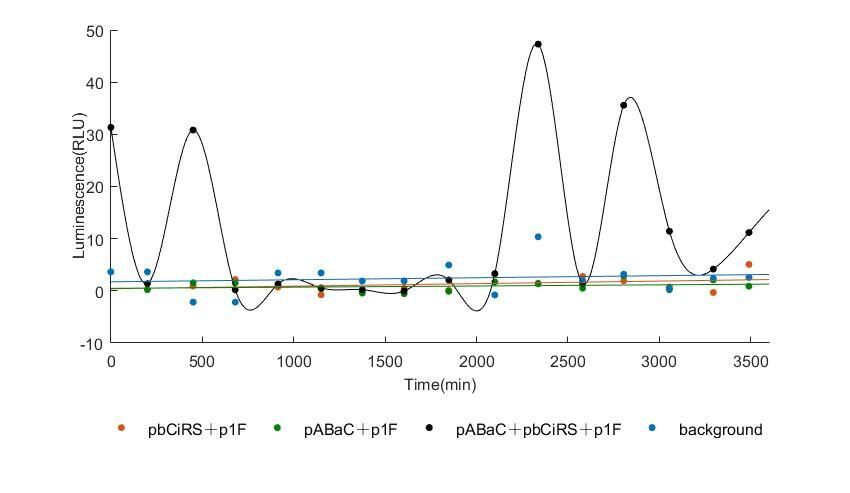
When we start to measure the datas of experiment group, we set up some control groups at the same time. To prove that our oscillator works surely because of the yeast two-hybrid system, we constructed some bacteria in which there are only one fusion protein of yeast two-hybrid system and a reporter gene or a combination. And we choose 3 or 4 reporter genes as parallel experiment groups.
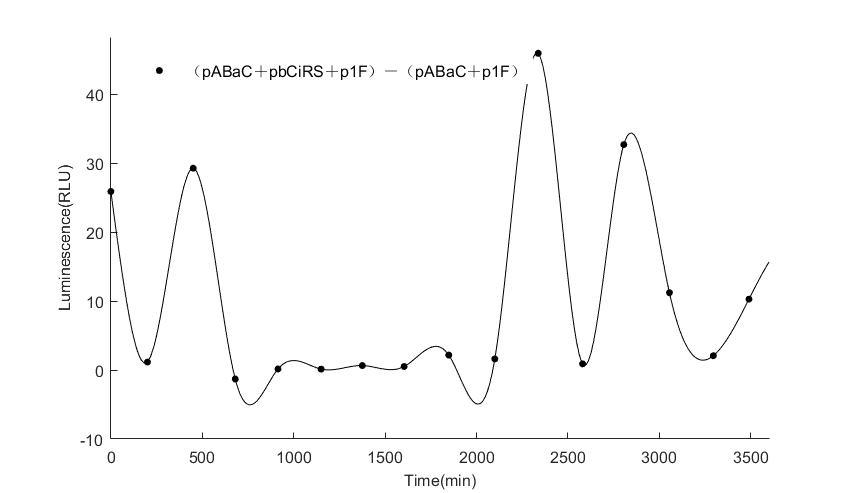
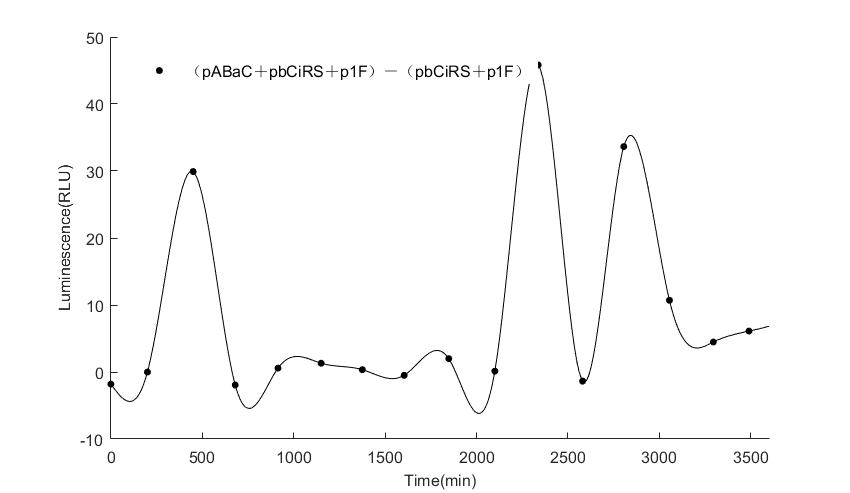
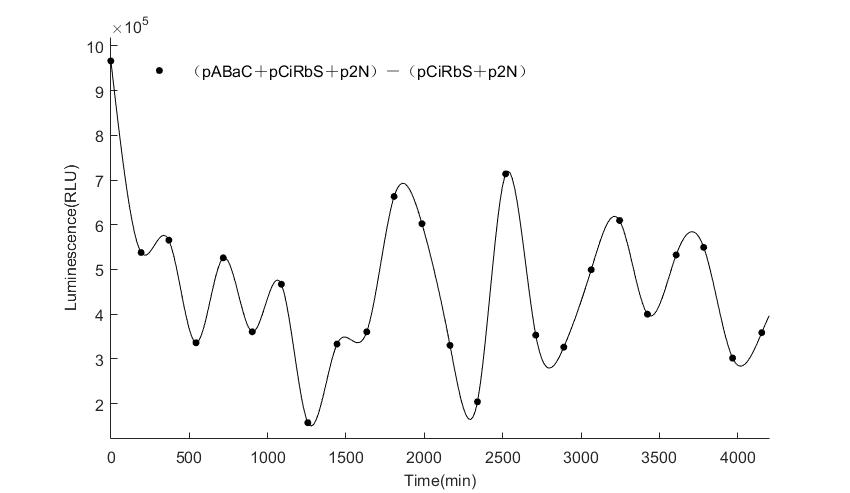
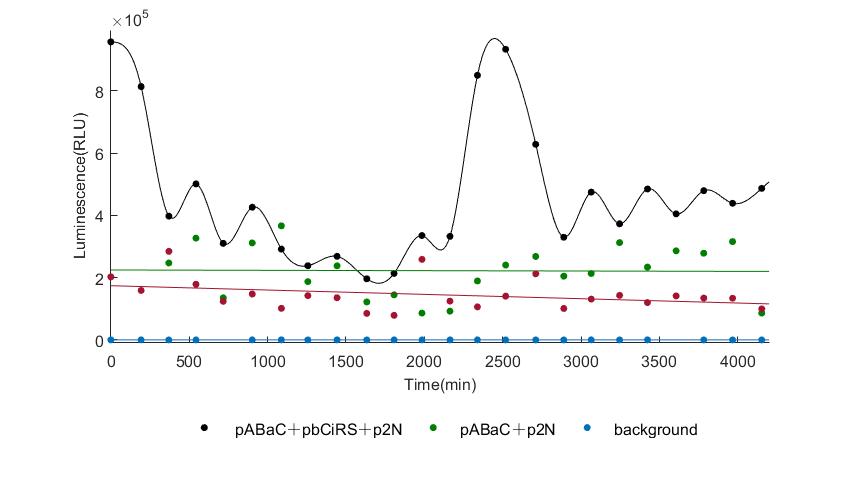
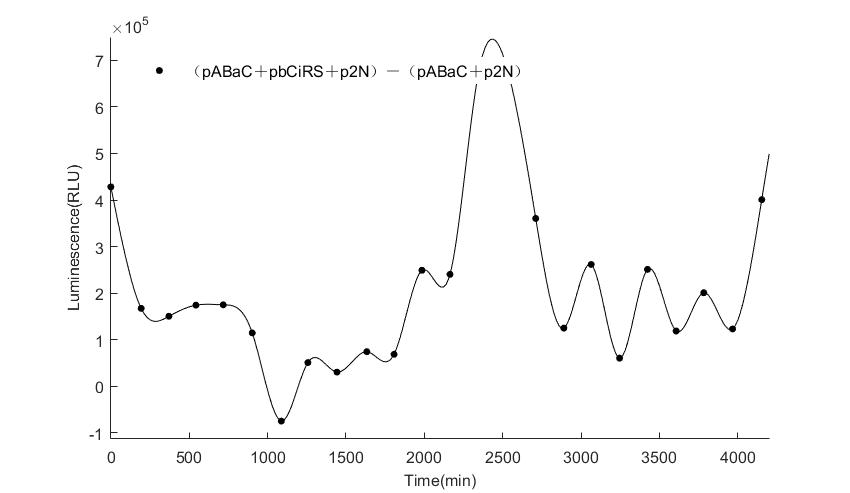
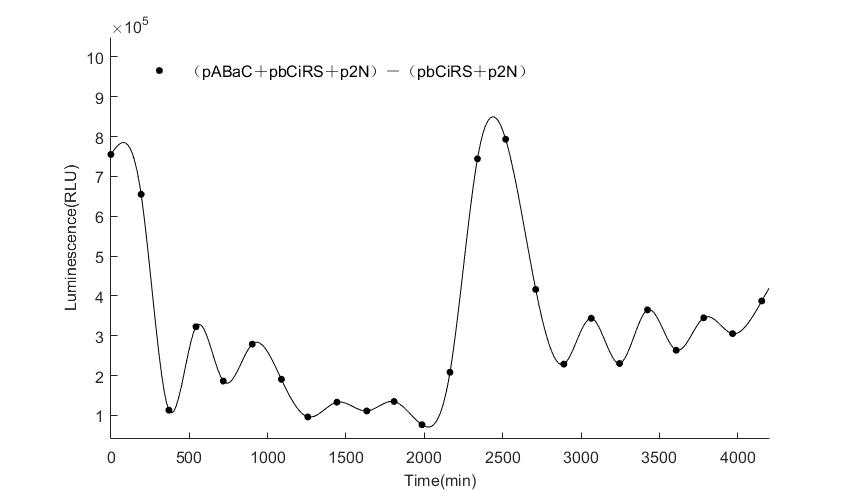
In the above figures, pABaC is the plasmid with AD-KaiC fused gene and make up a yeast two-hybrid system with the plasmid pbCiRS, which is with BD-CikA fused gene. p1F is the plasmid with Fluc reporter whose promoter is Gal1. In the 1st figure, the experimental group have black datas and the first control group and the second group have green and red datas, respectively. We substracted the control groups to gat a more objective result. As we predicted and looked at the results in other literatures, this oscillatory system is unstable.However, even though the fluctuations are not obvious on the second day, better waveforms could be observed on the first and third days.In addition, on the third day we get very high peak values, and in the wave trough experimental report gene expression is closer to the control group and the blank group, this indicates that in subjective day, yeast two-hybrid system works and results the peak value, in subjective night, it doesn't work and causes the similar results to the control groups and the background. Therefore, the overall experimental results show that we successfully reconstructed the oscillator in yeast.
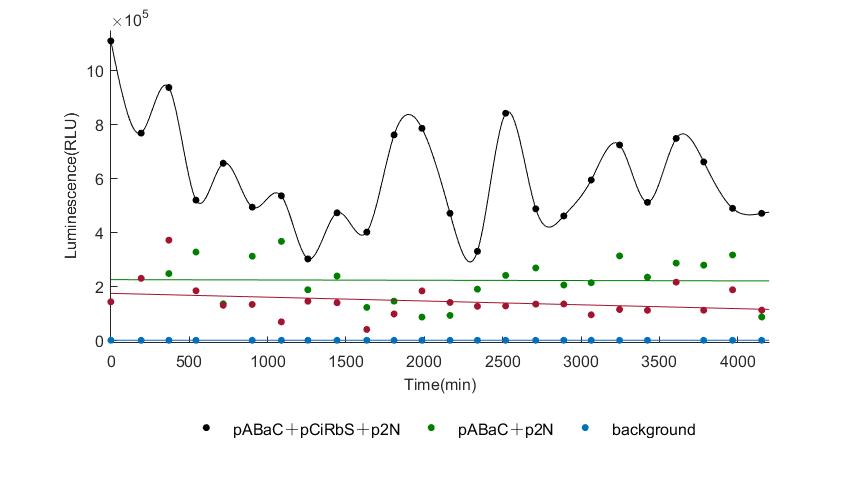
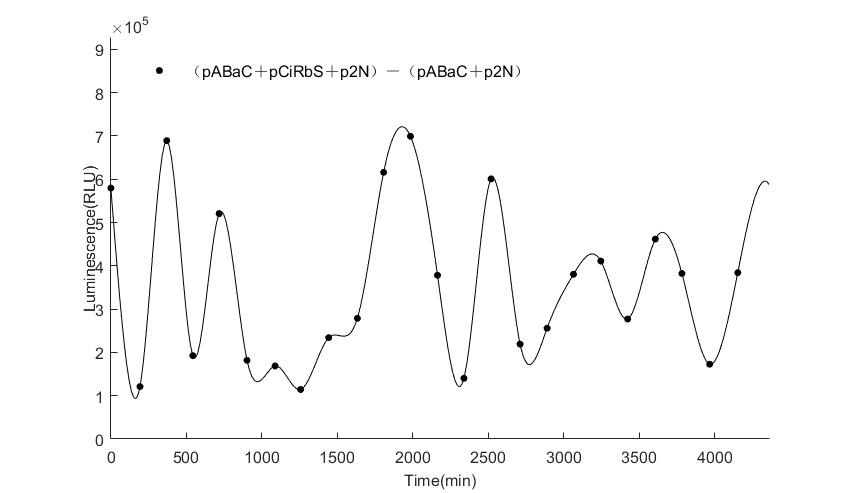
And the following figures are the results we measured with live Nanoluc. We get the similar conclusion to the Fluc group.
You can see more details of our results [http://2018.igem.org/Team:Tianjin/Demonstrate here]
References
[1]Roger Tseng, Nicolette F. Goularte, Archana Chavan, Jansen Luu, Susan E. Cohen, Yong-Gang Chang, Joel Heisler, Sheng Li, Alicia K. Michael, Sarvind Tripathi, Susan S. Golden, Andy LiWang, Carrie L. Partch, Structural basis of the day-night transition in a bacterial circadian clock. Science, 1174-1180 (2017).
[2]Joost Snijder, Jan M. Schuller, Anika Wiegard, Philip Lössl, Nicolas Schmelling, Ilka M. Axmann, Jürgen M. Plitzko, Friedrich Förster, Albert J. R. Heck, Structures of the cyanobacterial circadian oscillator frozen in a fully assembled state. Science, 1181-1184 (2017).
[3]Joseph S.Markson, Joseph R.Piechura, Anna M.Puszynska, Erin K.O’Shea, Circadian Control of Global Gene Expression by the Cyanobacterial Master Regulator RpaA. Cell, 1396-1408 (2013).
[4]Jun O. Liu,et al.Everything you need to know about the yeast two-hybrid system.[J]. Nature Structural Biology, 1998, 535-536
[5]Hideo Iwasaki,* Stanly B. Hideo, Iwasaki. Sensory Histidine Kinase, SasA, Necessary to Sustain Robust Circadian Oscillation in Cyanobacteria.[J]. cell, 2000, 101(2): 223-233