Difference between revisions of "Part:BBa K2558203"
(→Results) |
|||
| (4 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K2558203 short</partinfo> | <partinfo>BBa_K2558203 short</partinfo> | ||
| − | Tac promotor is a hybrid between trp and lacUV5 promoters. Tac promotor is a kind of strong E. coli | + | Tac promotor is a hybrid between trp and lacUV5 promoters. Tac promotor is a kind of strong E. coli promotor and is IPTG inducible. This device contains constitutively (BBa_J23100, Anderson strong promotor) expressed lacI protein and Ptac controlled sfGFP reporter. We designed this device along with K2558204 and K2558205, which are the same except for the strength of the constitutive lacI expression. We aim to specify the relation between the level of lacI production and the quality of IPTG induced expression. |
===Usage and Biology=== | ===Usage and Biology=== | ||
| − | In order to investigate how lacI dosage | + | In order to investigate how lacI dosage affects IPTG induction, we used Anderson promotor J23100, J23110 and J23114 to design three constitutive lacI generators of different intensities. The three lacI generators were then ligated with Ptac driven reporter sfGFP to make three IPTG induction devices (BBa_K2558203, BBa_K2558204, BBa_K2558205). By measuring sfGFP fluorescence we tested how these devices react to IPTG. |
===Results=== | ===Results=== | ||
| − | With high level of lacI expression ( | + | With high level of lacI expression (BBa_K2558203), sfGFP fluorescence had almost no response to IPTG induction. Weak lacI expression (BBa_K2558205) had the most significant IPTG induced sfGFP expression. With medium lacI expression level (BBa_K2558204), the induction efficiency lay in between. Therefore, the result proves that high level of lacI expression severely decreases IPTG induction efficiency [1]. Furthermore, IPTG concentration can affect the regulation part performance. The figure shows that without IPTG the sfGFP florescence intensity remained low. After IPTG addition, fluorescence signal immediately began to climb, forming a peak at five hours after induction, then sfGFP florescence intensity decreased and maintained at a lower level afterwards. IPTG concentration did not significantly affect the height of the peak or the expression level after the peak, but rather the peak width and expression stability of the system. Figures indicate that 5-10 mM IPTG had the most stable induction results. |
| − | [[File:T--Tsinghua-IPTGsfGFPresults.png|center | + | |
| + | <br/><li style="display: inline-block;"> [[File:T--Tsinghua-IPTGsfGFPresults-WithoutDescription.png|thumb|center|800px|'''Figure.1. The effect of varied lacI-LVA (BBa_C0011) promotor strength on IPTG induction of Tac promotor (BBa_K2558004).''' Relative fluorescent intensity is fluorescence per OD600 standardized with fluorescence per OD600 value of each test group at Time=0, IPTG=0. The promotors we used are from the Anderson collection: BBa_J23100 for strong lacI-LVA expression (pink), BBa_J23110 for medium expression (green), BBa_J23114 for weak expression (orange).]] | ||
<!-- --> | <!-- --> | ||
| − | |||
===Protocol=== | ===Protocol=== | ||
| − | # one Transform the plasmids into E. coli | + | # one Transform the plasmids into ''E. coli'' DH5α. |
# two Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 5ml M9 medium with ampicillin at 100 ng/µl. Incubate for 6-8 h at 37℃ in a shaker. | # two Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 5ml M9 medium with ampicillin at 100 ng/µl. Incubate for 6-8 h at 37℃ in a shaker. | ||
# three Measure OD600 of the culture medium with photometer. Dilute the culture medium until OD600 reaches 0.6. | # three Measure OD600 of the culture medium with photometer. Dilute the culture medium until OD600 reaches 0.6. | ||
# four Add 100 µl bacteria culture medium into a sterile 96-well plate. Add IPTG to final concentrations of 0, 1, 5, 10, 20 mM. Fresh M9 medium serves as blank control. Positive control is colony constantly expressing sfGFP and negative control is colony without sfGFP expression. Place the 96-well plate into an automatic microplate reader. Incubate at 16℃ overnight and record the fluorometric value at 510 nm and OD600 for each well every 30 minutes. | # four Add 100 µl bacteria culture medium into a sterile 96-well plate. Add IPTG to final concentrations of 0, 1, 5, 10, 20 mM. Fresh M9 medium serves as blank control. Positive control is colony constantly expressing sfGFP and negative control is colony without sfGFP expression. Place the 96-well plate into an automatic microplate reader. Incubate at 16℃ overnight and record the fluorometric value at 510 nm and OD600 for each well every 30 minutes. | ||
| − | # five Each group | + | # five Each group should be repeated for at least 3 times. |
| − | + | ||
===Reference=== | ===Reference=== | ||
[1] Szabolcs Semsey, Sandeep Krishna. "The effect of LacI autoregulation on the performance of the lactose utilization system in Escherichia coli" Nucleic Acids Res 2013 Jul; 41(13): 6381–6390 | [1] Szabolcs Semsey, Sandeep Krishna. "The effect of LacI autoregulation on the performance of the lactose utilization system in Escherichia coli" Nucleic Acids Res 2013 Jul; 41(13): 6381–6390 | ||
| − | + | ===Sequence and Features=== | |
<partinfo>BBa_K2558203 SequenceAndFeatures</partinfo> | <partinfo>BBa_K2558203 SequenceAndFeatures</partinfo> | ||
Latest revision as of 16:18, 17 October 2018
Ptac test device with Anderson strong-expressed lacI
Tac promotor is a hybrid between trp and lacUV5 promoters. Tac promotor is a kind of strong E. coli promotor and is IPTG inducible. This device contains constitutively (BBa_J23100, Anderson strong promotor) expressed lacI protein and Ptac controlled sfGFP reporter. We designed this device along with K2558204 and K2558205, which are the same except for the strength of the constitutive lacI expression. We aim to specify the relation between the level of lacI production and the quality of IPTG induced expression.
Usage and Biology
In order to investigate how lacI dosage affects IPTG induction, we used Anderson promotor J23100, J23110 and J23114 to design three constitutive lacI generators of different intensities. The three lacI generators were then ligated with Ptac driven reporter sfGFP to make three IPTG induction devices (BBa_K2558203, BBa_K2558204, BBa_K2558205). By measuring sfGFP fluorescence we tested how these devices react to IPTG.
Results
With high level of lacI expression (BBa_K2558203), sfGFP fluorescence had almost no response to IPTG induction. Weak lacI expression (BBa_K2558205) had the most significant IPTG induced sfGFP expression. With medium lacI expression level (BBa_K2558204), the induction efficiency lay in between. Therefore, the result proves that high level of lacI expression severely decreases IPTG induction efficiency [1]. Furthermore, IPTG concentration can affect the regulation part performance. The figure shows that without IPTG the sfGFP florescence intensity remained low. After IPTG addition, fluorescence signal immediately began to climb, forming a peak at five hours after induction, then sfGFP florescence intensity decreased and maintained at a lower level afterwards. IPTG concentration did not significantly affect the height of the peak or the expression level after the peak, but rather the peak width and expression stability of the system. Figures indicate that 5-10 mM IPTG had the most stable induction results.
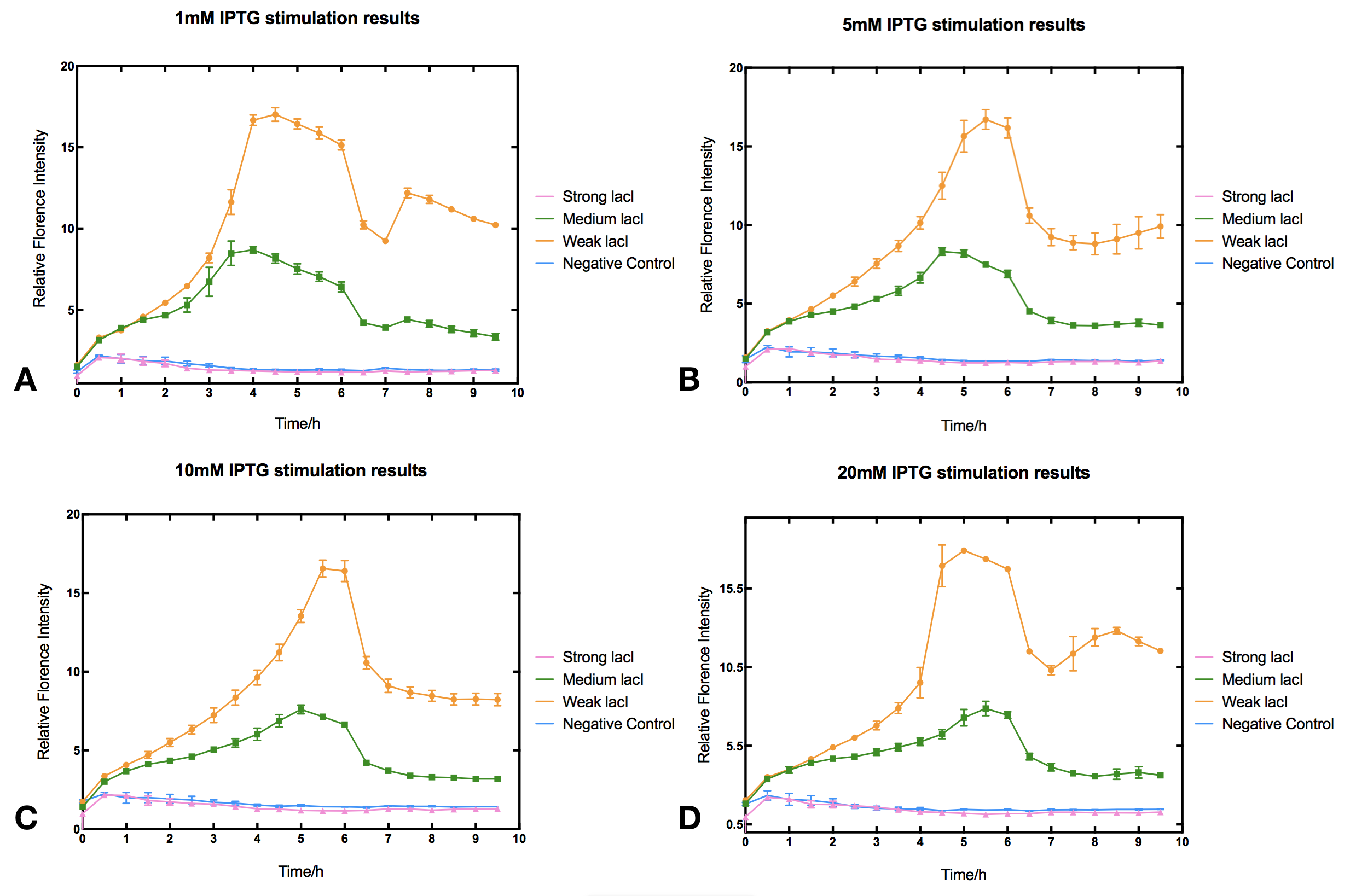
Protocol
- one Transform the plasmids into E. coli DH5α.
- two Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 5ml M9 medium with ampicillin at 100 ng/µl. Incubate for 6-8 h at 37℃ in a shaker.
- three Measure OD600 of the culture medium with photometer. Dilute the culture medium until OD600 reaches 0.6.
- four Add 100 µl bacteria culture medium into a sterile 96-well plate. Add IPTG to final concentrations of 0, 1, 5, 10, 20 mM. Fresh M9 medium serves as blank control. Positive control is colony constantly expressing sfGFP and negative control is colony without sfGFP expression. Place the 96-well plate into an automatic microplate reader. Incubate at 16℃ overnight and record the fluorometric value at 510 nm and OD600 for each well every 30 minutes.
- five Each group should be repeated for at least 3 times.
Reference
[1] Szabolcs Semsey, Sandeep Krishna. "The effect of LacI autoregulation on the performance of the lactose utilization system in Escherichia coli" Nucleic Acids Res 2013 Jul; 41(13): 6381–6390
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 1073
Illegal NheI site found at 1096 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 2277
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 176
