Difference between revisions of "Part:BBa K2541304"
| (5 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K2541304 short</partinfo> | <partinfo>BBa_K2541304 short</partinfo> | ||
| + | <h5> | ||
| + | <P style="text-indent:2em;"> | ||
| + | A RNA-based thermosensor that can be used for temperature sensitive translational regulation which is based on the change of RNA advanced structure. The cold-inducible RNA-based thermosensors can initiate translation of downstream genes at low temperatures. | ||
| + | </p> | ||
| + | </h5> | ||
| − | + | <h1>'''1. Usage and Biology'''</h1> | |
| + | <h5> | ||
| + | <P style="text-indent:2em;"> | ||
| + | There are multiple families of cold-inducible proteins in prokaryotes, the most widely studied of which are the Csp family of cold shock proteins in ''E.coli''. CspA represents CspA family, which has been quite extensively studied for the mechanism of its cold response. There is a temperature-sensing region in the 5’untranslated region (5'UTR) of ''CspA'' mRNA, which can regulate the accessibility of the translation initiation region by altering the advanced structure of RNA, thereby regulating the initiation of translation. | ||
| + | </p> | ||
| + | <P style="text-indent:2em;"> | ||
| + | At low temperatures (<20℃), 5’UTR of ''CspA'' mRNA can form an advanced structure called pseudoknot, which is more efficiently translated because the conformation exposes the Shine–Dalgarno (SD) sequence, it is beneficial to recruit ribosomes and somewhat less susceptible to degradation. At normal temperatures, due to thermodynamic instability, pseudoknot unfolds. 5’UTR forms a secondary structure masking SD sequence to block translation initiation region, which impedes translation. We designed a series of cold-inducible RNA-based thermosensors with different melting temperatures, intensity and sensitivity based on the pseudoknot structure. | ||
| + | </p> | ||
| + | <P style="text-indent:2em;"> | ||
| + | Our team designed synthetic cold-inducible RNA-based thermosensors that are considerably simpler than naturally occurring cspA thermosensors and can be exploited as convenient on/off switches of gene expression. | ||
| + | </p> | ||
| + | </h5> | ||
| + | <html><center> | ||
| + | <div class="stem-loop cold-induced"> | ||
| − | < | + | <input id="checked_3" type="checkbox" class="switch" /> |
| − | === | + | |
| + | <svg class="svg_animate" xmlns="http://www.w3.org/2000/svg" width="50%" viewBox="0 0 374.12 206.26"> | ||
| + | <defs> | ||
| + | <style> | ||
| + | |||
| + | .switch::before{ | ||
| + | content:""; | ||
| + | display:block; | ||
| + | height:2rem; | ||
| + | width:4rem; | ||
| + | position:absolute; | ||
| + | } | ||
| + | .switch{ | ||
| + | display:block; | ||
| + | height:2rem; | ||
| + | width:4rem; | ||
| + | opacity:1; | ||
| + | border-radius:2rem; | ||
| + | background: #00B8FF; | ||
| + | -webkit-appearance: none; | ||
| + | cursor:pointer; | ||
| + | border:0; | ||
| + | position:relative; | ||
| + | transition:all 0.3s ease; | ||
| + | margin:2rem; | ||
| + | } | ||
| + | .switch::after{ | ||
| + | content:""; | ||
| + | position:absolute; | ||
| + | display:block; | ||
| + | height:2rem; | ||
| + | width:2rem; | ||
| + | border-radius:2rem; | ||
| + | background: white; | ||
| + | box-shadow: 0 0 1rem rgba(0,0,0,0.2); | ||
| + | box-sizing:border-box; | ||
| + | transition:all 0.3s ease; | ||
| + | transform:translateX(0rem); | ||
| + | } | ||
| + | .switch:checked::after{ | ||
| + | transform:translateX(2rem); | ||
| + | } | ||
| + | .switch:checked{ | ||
| + | background: #FFAB63; | ||
| + | } | ||
| + | |||
| + | .cold-induced .a,.cold-induced .b,.cold-induced .c { | ||
| + | fill: none; | ||
| + | stroke-linecap: round; | ||
| + | stroke-linejoin: round; | ||
| + | stroke-width: 11px; | ||
| + | } | ||
| + | .cold-induced .a { | ||
| + | stroke: #036; | ||
| + | } | ||
| + | .cold-induced .b { | ||
| + | stroke: #060; | ||
| + | } | ||
| + | .cold-induced .c { | ||
| + | stroke: #600; | ||
| + | } | ||
| + | .cold-induced .l{ | ||
| + | fill: none; | ||
| + | stroke-linecap: round; | ||
| + | stroke-linejoin: round; | ||
| + | stroke: #000; | ||
| + | stroke-width: 11px; | ||
| + | } | ||
| + | .hydrogen-bond { | ||
| + | opacity: 1; | ||
| + | transition: all 0.3s ease 1s; | ||
| + | } | ||
| + | .rotate{ | ||
| + | transition: all 1s ease; | ||
| + | transform: rotate(90deg); | ||
| + | } | ||
| + | .r1{ | ||
| + | transform-origin: 41% 85.7%; | ||
| + | transition-delay:0.5s; | ||
| + | } | ||
| + | .r2{ | ||
| + | transform-origin: 54% 14.5%; | ||
| + | } | ||
| + | .cold-induced.svg_checked .rotate{ | ||
| + | transform: rotate(30deg); | ||
| + | } | ||
| + | .cold-induced.svg_checked .hydrogen-bond{ | ||
| + | opacity:0; | ||
| + | transition: all 0.3s ease 0s; | ||
| + | } | ||
| + | |||
| + | </style> | ||
| + | </defs> | ||
| + | <g> | ||
| + | <path class="l rotate r1" d="M177.06,176.54a24.23,24.23,0,0,1-24.23,24.22H5.5"/> | ||
| + | <path class="l" d="M201.28,5.5a24.22,24.22,0,0,0-24.22,24.22V176.54a24.23,24.23,0,0,1-24.23,24.22"/> | ||
| + | <path class="l rotate r2" d="M348.62,5.5H201.28a24.22,24.22,0,0,0-24.22,24.22"/> | ||
| + | <g class="hydrogen-bond"> | ||
| + | <line class="a" x1="219.21" y1="86.31" x2="183.35" y2="86.31"/> | ||
| + | <line class="b" x1="219.21" y1="67.42" x2="183.35" y2="67.42"/> | ||
| + | <line class="c" x1="219.21" y1="48.54" x2="183.35" y2="48.54"/> | ||
| + | </g> | ||
| + | <g class="hydrogen-bond"> | ||
| + | <line class="a" x1="170.76" y1="150.31" x2="134.9" y2="150.31"/> | ||
| + | <line class="b" x1="170.76" y1="169.2" x2="134.9" y2="169.2"/> | ||
| + | <line class="c" x1="170.76" y1="131.42" x2="134.9" y2="131.42"/> | ||
| + | <line class="a" x1="170.76" y1="112.54" x2="134.9" y2="112.54"/> | ||
| + | </g> | ||
| + | </g> | ||
| + | <p>Figure 1. Mechanism of cold-inducible RNA-based thermosensors.</p> | ||
| + | </svg> | ||
| + | |||
| + | |||
| + | <script type="text/javascript"> | ||
| + | $(document).ready(function(){ | ||
| + | $("#checked_3").click(function(){ | ||
| + | if($(this).is(':checked')){ | ||
| + | $(".cold-induced").addClass("svg_checked"); | ||
| + | }else{ | ||
| + | $(".cold-induced").removeClass("svg_checked"); | ||
| + | } | ||
| + | }); | ||
| + | }); | ||
| + | </script> | ||
| + | </div></center> | ||
| + | </html> | ||
| + | |||
| + | <h1>'''2. Design'''</h1> | ||
| + | <h5> | ||
| + | <P style="text-indent:2em;"> | ||
| + | In our design, we deleted the conserved region called the cold box upstream of the 5'UTR of ''CspA'' mRNA, so that the expression of CspA is not regulated by its own negative feedback. The pseudoknot in the ''CspA'' mRNA contains four sets of base pairings, and its stability is temperature-regulated. Several structural parameters come into consideration to optimize cold-inducible RNA-based thermosensors: base pairing, base pair position and GC content in pseudoknot region. Increasing base pairing can raise the melting temperature of pseudoknot, thus enlarging the temperature range of open conformation. Increasing GC content can also raise the melting temperature of pseudoknot. We also change base pair at different position. What’s more, we delete the cold box which is at the upstream of ''CspA'' 5’UTR. In K2541304, we increased the GC content by adding two base pairs (G:C) in the pseudoknot region (figure 2). | ||
| + | </p> | ||
| + | </h5> | ||
| + | |||
| + | [[File:K2541304 f2.png|center|K2541304 f2]] | ||
| + | <center>Figure 2. Design of K2541304.</center> | ||
| + | |||
| + | <h1>'''3.Characterization'''</h1> | ||
| + | <h3>3.1 Measurement device</h3> | ||
| + | <h5> | ||
| + | <P style="text-indent:2em;"> | ||
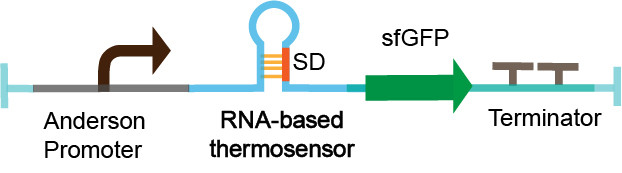
| + | The thermosensor sequence is constructed on the pSB1C3 vector by Golden Gate assembly. The measurement device is composed of Anderson promoter (BBa_J23100), thermosensor (BBa_K2541304), sfGFP_optimism (BBa_K2541400) and double terminator (BBa_B0010 and BBa_B0012). We select a constitutive Anderson promoter J23100 as an appropriate promoter by pre-experiment. The sfGFP_optimism has faster folding speed and higher fluorescence intensity. The double terminator can reduce leakage (Figure 3). We characterized RNA-based thermosensors in ''E.coli'' DH5a. | ||
| + | </p> | ||
| + | </h5> | ||
| + | [[File:measurement device 123.png|center|caption]] | ||
| + | <center>Figure 3. The measurement device.</center> | ||
| + | ---- | ||
| + | |||
| + | <h3>3.2 Measurement results</h3> | ||
| + | <h5> | ||
| + | <P style="text-indent:2em;"> | ||
| + | In figure 4, there are eight different cold-inducible RNA-based thermosensors. pos.control is positive control. The final normalized fluorescence was calculated as follows: normalized fluorescence = [(Fluorescence/Abs<sub>600</sub>)<sub>TS</sub> - (Fluorescence/Abs<sub>600</sub>)<sub>neg</sub>] / [(Fluorescence/Abs<sub>600</sub>)<sub>pos</sub> - (Fluorescence/Abs<sub>600</sub>)<sub>neg</sub>] ( TS = thermosensor, pos = positive control, and neg = BBa_J364007 ). As shown in figure 4, the fluorescence intensity of K2541304 increases with decreased temperature. | ||
| + | </p> | ||
| + | </h5> | ||
| + | [[File:K2541304 f4.png|center|K2541304 f4]] | ||
| + | Figure 4. Characteristics of K2541304. Each set of three bars represents the activity level of a different thermosensor. The bar colors purple, yellow and red represent the temperatures 15, 20 and 25°C, respectively. The height of the bars corresponds to the normalized fluorescence. | ||
| + | |||
| + | <h1>'''4. Collection of cold-inducible RNA-based thermosensors '''</h1> | ||
| + | [[File:cspA figure6 new.png|center|cspA figure6 new]] | ||
| + | Figure 5. Experimental measurements of the collection of cold-inducible RNA-based thermosensors show a variety of responses. (A) Rows represent activity levels of different thermosensors. (B) Each set of three bars represents the activity level of a different thermosensor. The bar colors purple, yellow and red represent the temperatures 15, 20 and 25°C, respectively. The height of the bars corresponds to the normalized fluorescence. | ||
| + | |||
| + | <h1>'''5. Conclusion'''</h1> | ||
| + | <h5> | ||
| + | <P style="text-indent:2em;"> | ||
| + | Our data show that efficient RNA-based thermosensors with different melting temperatures, intensity and sensitivity can be built from RNA advanced structure, thus providing useful SynRT toolkit for the regulation of gene expression. | ||
| + | </p> | ||
| + | </h5> | ||
| − | |||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K2541304 SequenceAndFeatures</partinfo> | <partinfo>BBa_K2541304 SequenceAndFeatures</partinfo> | ||
Latest revision as of 09:03, 16 October 2018
Cold-inducible RNA-based thermosensor-4
A RNA-based thermosensor that can be used for temperature sensitive translational regulation which is based on the change of RNA advanced structure. The cold-inducible RNA-based thermosensors can initiate translation of downstream genes at low temperatures.
1. Usage and Biology
There are multiple families of cold-inducible proteins in prokaryotes, the most widely studied of which are the Csp family of cold shock proteins in E.coli. CspA represents CspA family, which has been quite extensively studied for the mechanism of its cold response. There is a temperature-sensing region in the 5’untranslated region (5'UTR) of CspA mRNA, which can regulate the accessibility of the translation initiation region by altering the advanced structure of RNA, thereby regulating the initiation of translation.
At low temperatures (<20℃), 5’UTR of CspA mRNA can form an advanced structure called pseudoknot, which is more efficiently translated because the conformation exposes the Shine–Dalgarno (SD) sequence, it is beneficial to recruit ribosomes and somewhat less susceptible to degradation. At normal temperatures, due to thermodynamic instability, pseudoknot unfolds. 5’UTR forms a secondary structure masking SD sequence to block translation initiation region, which impedes translation. We designed a series of cold-inducible RNA-based thermosensors with different melting temperatures, intensity and sensitivity based on the pseudoknot structure.
Our team designed synthetic cold-inducible RNA-based thermosensors that are considerably simpler than naturally occurring cspA thermosensors and can be exploited as convenient on/off switches of gene expression.
2. Design
In our design, we deleted the conserved region called the cold box upstream of the 5'UTR of CspA mRNA, so that the expression of CspA is not regulated by its own negative feedback. The pseudoknot in the CspA mRNA contains four sets of base pairings, and its stability is temperature-regulated. Several structural parameters come into consideration to optimize cold-inducible RNA-based thermosensors: base pairing, base pair position and GC content in pseudoknot region. Increasing base pairing can raise the melting temperature of pseudoknot, thus enlarging the temperature range of open conformation. Increasing GC content can also raise the melting temperature of pseudoknot. We also change base pair at different position. What’s more, we delete the cold box which is at the upstream of CspA 5’UTR. In K2541304, we increased the GC content by adding two base pairs (G:C) in the pseudoknot region (figure 2).
3.Characterization
3.1 Measurement device
The thermosensor sequence is constructed on the pSB1C3 vector by Golden Gate assembly. The measurement device is composed of Anderson promoter (BBa_J23100), thermosensor (BBa_K2541304), sfGFP_optimism (BBa_K2541400) and double terminator (BBa_B0010 and BBa_B0012). We select a constitutive Anderson promoter J23100 as an appropriate promoter by pre-experiment. The sfGFP_optimism has faster folding speed and higher fluorescence intensity. The double terminator can reduce leakage (Figure 3). We characterized RNA-based thermosensors in E.coli DH5a.
3.2 Measurement results
In figure 4, there are eight different cold-inducible RNA-based thermosensors. pos.control is positive control. The final normalized fluorescence was calculated as follows: normalized fluorescence = [(Fluorescence/Abs600)TS - (Fluorescence/Abs600)neg] / [(Fluorescence/Abs600)pos - (Fluorescence/Abs600)neg] ( TS = thermosensor, pos = positive control, and neg = BBa_J364007 ). As shown in figure 4, the fluorescence intensity of K2541304 increases with decreased temperature.
Figure 4. Characteristics of K2541304. Each set of three bars represents the activity level of a different thermosensor. The bar colors purple, yellow and red represent the temperatures 15, 20 and 25°C, respectively. The height of the bars corresponds to the normalized fluorescence.
4. Collection of cold-inducible RNA-based thermosensors
Figure 5. Experimental measurements of the collection of cold-inducible RNA-based thermosensors show a variety of responses. (A) Rows represent activity levels of different thermosensors. (B) Each set of three bars represents the activity level of a different thermosensor. The bar colors purple, yellow and red represent the temperatures 15, 20 and 25°C, respectively. The height of the bars corresponds to the normalized fluorescence.
5. Conclusion
Our data show that efficient RNA-based thermosensors with different melting temperatures, intensity and sensitivity can be built from RNA advanced structure, thus providing useful SynRT toolkit for the regulation of gene expression.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]