Difference between revisions of "Part:BBa K2273035"
| (5 intermediate revisions by the same user not shown) | |||
| Line 21: | Line 21: | ||
| − | This composite part was used for evalutaion in the [http://2017.igem.org/Team:TU_Dresden/Project/Secretion secretion project] of 2017 TU_Dresden iGEM [http://2017.igem.org/Team:TU_Dresden team]. It codes for a fluorescent reporter protein ([https://parts.igem.org/Part:BBa_K2273034 mCherry]) and a functional tag ([https://parts.igem.org/Part:BBa_K2273016 SpyTag His-tagged]), mediating covalent bonding with | + | This composite part was used for evalutaion in the [http://2017.igem.org/Team:TU_Dresden/Project/Secretion secretion project] of 2017 TU_Dresden iGEM [http://2017.igem.org/Team:TU_Dresden team]. It codes for a fluorescent reporter protein ([https://parts.igem.org/Part:BBa_K2273034 mCherry]) and a functional tag ([https://parts.igem.org/Part:BBa_K2273016 SpyTag His-tagged]), mediating covalent bonding with its tag partner ([https://parts.igem.org/Part:BBa_K2273015 SpyCatcher]). It is optimized for usage in <i>Bacillus subtilis</i>. |
===Design=== | ===Design=== | ||
| Line 29: | Line 29: | ||
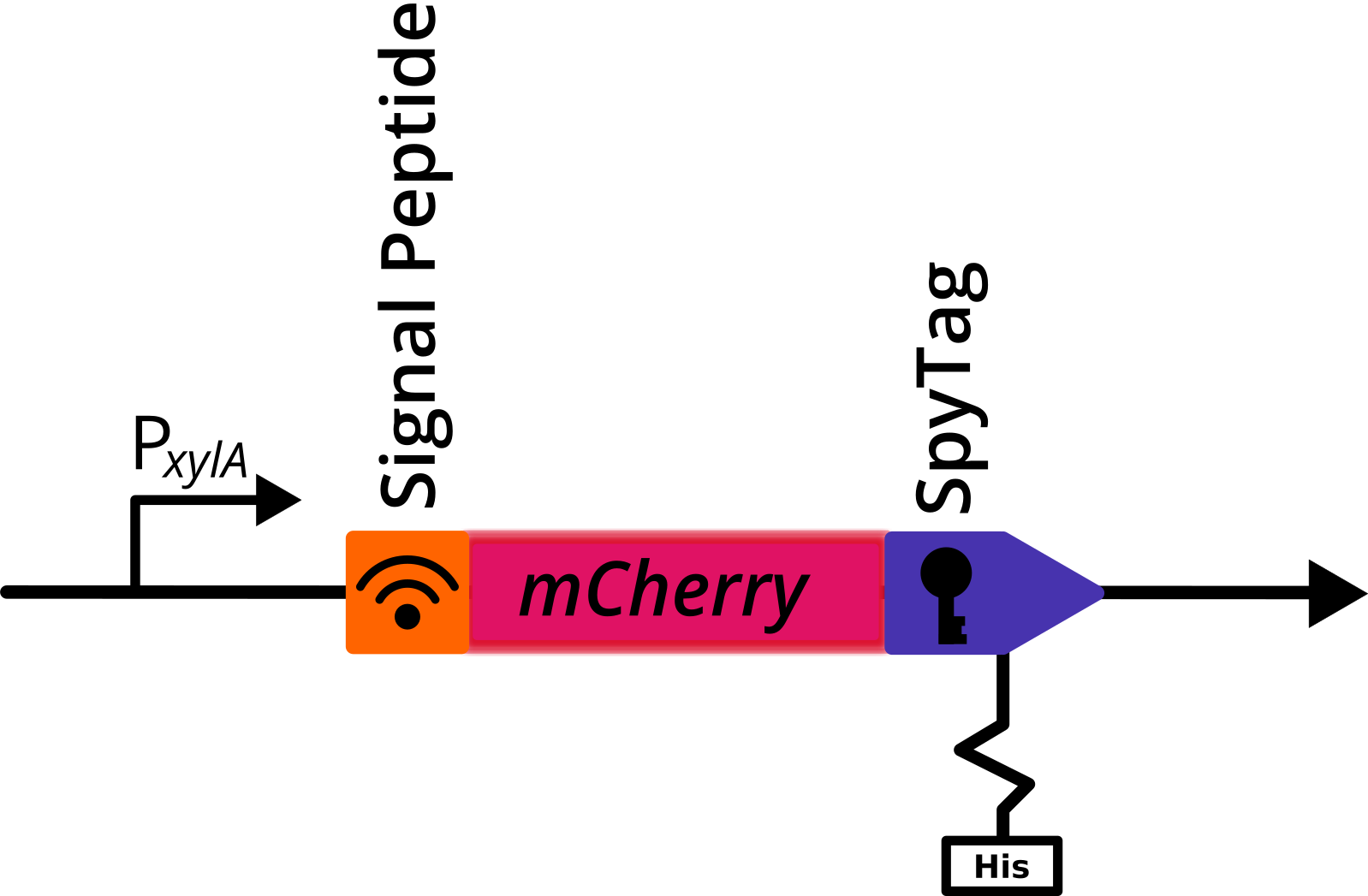
A signal peptide ([https://parts.igem.org/Part:BBa_K2273023 AmyE SP]) was fused n-terminally to this construct to induce secretion. | A signal peptide ([https://parts.igem.org/Part:BBa_K2273023 AmyE SP]) was fused n-terminally to this construct to induce secretion. | ||
| − | [[File:MCherry tag.png|thumb|centre|600px|'''Figure 1:''': Genetic | + | [[File:MCherry tag.png|thumb|centre|600px|'''Figure 1:''': Genetic construct with mCherry. Depicted is a translational fusion constructs downstream of the <i>PxylA</i> promotor, that was cloned in the multiple cloning site of the pBS2E<i><sub>PxylA</sub></i> vector. The construct contains a signal peptide sequence, the gene coding for mCherry, either c-terminally fused SpyTag and a his-tag.]] |
| Line 47: | Line 47: | ||
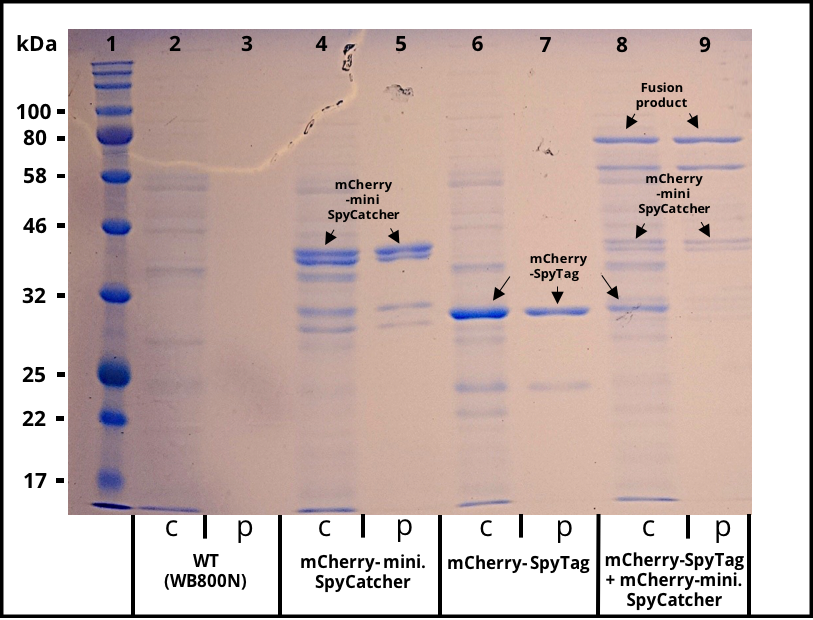
[[File:T--TU_Dresden--secretion---result--sds.png|thumb|left|350px|'''Figure 3:''' SDS gel with crude and purified supernatants. Expression of the multi copy mCherry constructs was induced with 1% Xylose and the supernatants were harvested after 16 h of incubation. The his-tagged proteins were purified with Ni-NTA agarose beads. Lane 1 was loaded with 3 µl of NEB´s “Color Prestained Protein Standard Broad Range” ladder. Crude (c) and purified (p) supernatant of wild-type (WT) are shown as a control in lane 2 and 3. Lane 4 and 5 contain the supernatant of B. subtilis producing mCherry-mini. SpyCatcher fusion protein (36,6 kDa). Lane 4 and 5 contain the supernatant of B. subtilis producing mCherry-SpyTag fusion protein (31,9 kDa). The crude supernatants of the two mCherry producing strains were combined, incubated for 4 h, purified and loaded onto lane 8 and 9. The fusion product of the mCherry constructs is visable in the crude and purified supernatant.]] | [[File:T--TU_Dresden--secretion---result--sds.png|thumb|left|350px|'''Figure 3:''' SDS gel with crude and purified supernatants. Expression of the multi copy mCherry constructs was induced with 1% Xylose and the supernatants were harvested after 16 h of incubation. The his-tagged proteins were purified with Ni-NTA agarose beads. Lane 1 was loaded with 3 µl of NEB´s “Color Prestained Protein Standard Broad Range” ladder. Crude (c) and purified (p) supernatant of wild-type (WT) are shown as a control in lane 2 and 3. Lane 4 and 5 contain the supernatant of B. subtilis producing mCherry-mini. SpyCatcher fusion protein (36,6 kDa). Lane 4 and 5 contain the supernatant of B. subtilis producing mCherry-SpyTag fusion protein (31,9 kDa). The crude supernatants of the two mCherry producing strains were combined, incubated for 4 h, purified and loaded onto lane 8 and 9. The fusion product of the mCherry constructs is visable in the crude and purified supernatant.]] | ||
| + | |||
| + | |||
| + | |||
| + | <partinfo>BBa_K2273035 SequenceAndFeatures</partinfo> | ||
Latest revision as of 19:07, 1 November 2017
| Part Information | |
|---|---|
| RFC standard | RFC 25 |
| Fused Tag | BBa_K2273016: Spytag His-tagged |
| Fluorescent Protein | BBa_K2273034: mCherry |
| Submitted by | [http://2017.igem.org/Team:TU_Dresden TU Dresden] |
mCherry with C-Terminal SpyTag and His Tag
This composite part was used for evalutaion in the [http://2017.igem.org/Team:TU_Dresden/Project/Secretion secretion project] of 2017 TU_Dresden iGEM [http://2017.igem.org/Team:TU_Dresden team]. It codes for a fluorescent reporter protein (mCherry) and a functional tag (SpyTag His-tagged), mediating covalent bonding with its tag partner (SpyCatcher). It is optimized for usage in Bacillus subtilis.
Design
A signal peptide (AmyE SP) was fused n-terminally to this construct to induce secretion.
Application
The successful secretion could be proven with a fluorescence assay using the supernatants of B. subtilis (Figure 2). The functionality of the SpyTag/SpyCatcher was proven via SDS-PAGE, using the supernatants (Figure 3).
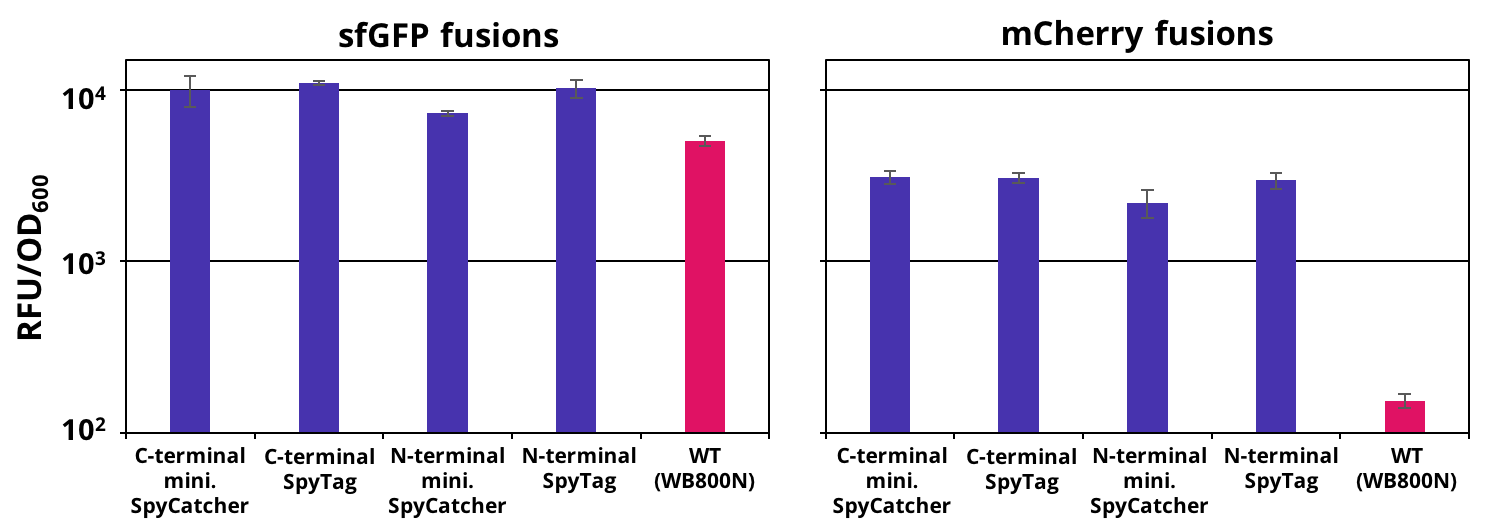
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
