Difference between revisions of "Part:BBa K2406020"
JackSuitor (Talk | contribs) |
|||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 17: | Line 17: | ||
==Sequences== | ==Sequences== | ||
Find sequences of all promoter-regulated generators and measurement constructs here: [[Media:File:Sequencing Results Edinburgh UG.zip]] | Find sequences of all promoter-regulated generators and measurement constructs here: [[Media:File:Sequencing Results Edinburgh UG.zip]] | ||
| + | ==Team Tec-Monterrey characterization== | ||
| + | While we were working in the expression of our chimeric proteins we realized that the promoter has an important leakage. | ||
| + | |||
| + | We first noticed visually by noticeable fluorescence on non-induced bacteria. | ||
| + | <div class = "left"> | ||
| + | https://2019.igem.org/wiki/images/thumb/a/a9/T--Tec-Monterrey--nul-2.1.jpg/800px-T--Tec-Monterrey--nul-2.1.jpg | ||
| + | </div> | ||
| + | Figure 1. 200µM IPTG induced sfGFP transformed bacteria (note that it flouresces less than the non-induced one). | ||
| + | |||
| + | <div class = "left"> | ||
| + | https://2019.igem.org/wiki/images/thumb/5/53/T--Tec-Monterrey--nufo-2.1.jpg/800px-T--Tec-Monterrey--nufo-2.1.jpg | ||
| + | </div> | ||
| + | Figure 2. non-induced E.coli. | ||
| + | |||
| + | We confirmed the leakage with a fluorescence measurement, where bacteria were growth at 30ºC for 3 hrs. Then 100µl of the grown bacteria were induced with IPTG at 0, 40, 200 and 400 mMm microtiter florescent measures were taken every half an hour for a period of 3 hours. Absorption and emission values were 395/509 nm. | ||
| + | |||
| + | <div class = "left"> | ||
| + | https://2019.igem.org/wiki/images/thumb/3/30/T--Tec-Monterrey--demostrate-grafica.png/800px-T--Tec-Monterrey--demostrate-grafica.png | ||
| + | </div> | ||
| + | Figure 3. sfGFP grpah, fluorescence vs time induced at different concentrations. | ||
| + | |||
| + | <div class = "left"> | ||
| + | https://2019.igem.org/wiki/images/thumb/3/32/T--Tec-Monterrey--part-sfGFP-sial-.png/800px-T--Tec-Monterrey--part-sfGFP-sial-.png | ||
| + | </div> | ||
| + | Figure 4. sial+sfGFP grpah, fluorescence vs time induced at different concentrations. | ||
| + | |||
| + | NOTE: We suggest that the lower fluorescence values in the induction of the sfGFP+sialidase than the sfGFP itself are due to the different sizes of the chimeric proteins that could difficult its secretion. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | =<b>Improvement By BJU_China iGEM2021</b>= | ||
| + | ==Description== | ||
| + | |||
| + | T7 promoter is commonly used in iGEM projects, [[Part:BBa_K2406020|BBa_K2406020]] designed by iGEM17_Edinburgh_UG was an inducile T7-LacO promoter for gene expression. In our project, we also used T7-LacO promoter to control the expression of halogenase. However, we found high basic leakage of this promoter, which affected the results of our experiments, so we improved this promoter in our project. | ||
| + | It is recognized that an excellent promoter should possess exceptional switch ratio: it should have lower background leakage in the "off" state and have obvious expression in the "on" state. In order to reduce the leakage and to ensure the repressor can bind better to the LacO site, we added another LacO site in the T7-LacO promoter as the figure below shows: | ||
| + | [[File:T--BJU_China--Fig.1 mprovement.png|600px|thumb|center]] | ||
| + | <p class="figure-description"><b><center>Fig.1 Improved promoter and original promoter</center></b></p> | ||
| + | |||
| + | ==Gene Circuit and Test Method== | ||
| + | |||
| + | In the experiment, LacI was designed as a repressor and IPTG as an inducer to control the state of T7 promoter. The gene circuit diagram is shown in Fig. 2. At the same time, we use sfGFP as reporter gene, so that the fluorescence value can be used to quantitatified gene expression level. The two gene circuits were constructed on pET28b vector and transformed to <i>E.coli</i> BL21(DE3). | ||
| + | [[File:T--BJU_China--Fig.2 improvement gene circuit.png|600px|thumb|center]] | ||
| + | <p class="figure-description"><b><center>Fig.2 Gene circuit for promoter test</center></b></p> | ||
| + | Single colonies of two bacteria (control, improved) were inoculated into LB medium, and cultivated vernight at 37° as seed culture. The seed culture was inoculated into M9 medium as 1:100, and IPTG were added at 0h respectively. The concentration of IPTG was 0, 10, 20, 50, 100, 200, 500, 1000 μM, and three parallel samples for each concentration. 200 μl of each sample were taken to measure the fluorescence after 6 hours of induction. | ||
| + | |||
| + | ==Results and Discussion== | ||
| + | |||
| + | From the results shown in Fig.3,we can see that the improved T7-LacO promoter is much better than the control group in terms of lower basic leakage or higher expression status. At the zero point,that is without inducer, the leakage of improved promoter is more than ten times lower than the control group. While when enough inducer exist, that is, when the gene circuit is fully turned on, the maximum expression of the optimized promoter is not suppressed, and even slightly higher than the control group. | ||
| + | Furthermore, when comparing the switch ratios of the two inducible promoters, the ratio of the control group was only about 12, while the switch ratio of improved promoter could reach 147, which also shows that our modification of this part is effective. | ||
| + | [[File:T--BJU_China--Fig.3 mprovement results.png|600px|thumb|center]] | ||
| + | <p class="figure-description"><b><center>Fig.3 Performance of improved promoter and original promoter</center></b></p> | ||
| + | In the above experiments and results, we optimized the characterization performance of the T7-LacO promoter by adding an extra LacO site. This may be because in the lactose operon model, the LacI repressor is a tetramer that binds to the LacO site to control the state of inducible promoter. The function of LacO involves various factors such as DNA bending and interaction between protein and DNA. The specific influence mechanism of the amount of lacO on the promoter needs to be further studied. | ||
| + | |||
| + | |||
| + | ==Team Uni-Hamburg 2022 characterization== | ||
| + | We wanted to use the T7 promoter to get an inducible control for different levels of fluorescence. We used the promoter together with BBa_E0034, BBa_E0040 and BBa_B0015 to build the inducible GFP unit. We then assembled it into the pSB1K3 backbone using goldengate assembly and transformed it into E.coli DH5alpha. | ||
| + | |||
| + | At this step we noticed high fluorescence even in the uninduced state. | ||
| + | <div class = "left"> | ||
| + | https://static.igem.wiki/teams/4472/wiki/parts/gfp-plate.png | ||
| + | </div> | ||
| + | Figure 1. Saving plate after colony PCR. Uninduced colonies containing the plasmid with the inducible GFP are clearly fluorescent while the plasmids not containing any GFP stay dark. | ||
| + | |||
| + | |||
| + | We further explored the leakage using a Tecan platereader with excitation at 485nm and emission at 515nm. The bacteria where cultured in 2 ml LB medium with kanamycin at 37°C for 6 hrs and then 250µl taken into a 96 well plate. We measured a serial 1:5 dilution. While cultures not containing the inducible GFP plasmid showed no fluorescence above background we could clearly observe fluorescence of the uninduced E.coli with the GFP plasmid. At a dilution of 1:25, even the GFP plasmids are not visible anymore as there are nearly no bacteria left. | ||
| + | [[File:GFP_uninduced.png|800px|thumb|left|Figure 2: Uninduced measurement of T7+LacO Promoter construct with GFP normalized against the negative control (bacteria without GFP construct)]] | ||
| + | |||
Latest revision as of 10:32, 12 October 2022
T7-LacO Promoter
Introduction
T7 Promoters are promoters that work with T7 RNA Polymerase. T7 RNA Polymerase is commonly used in biotechnology, as evidenced by the large number of parts found in the iGEM registry. T7 Polymerase has high affinity for its associated promoters and was identified as a useful tool for producing high amounts of RNA back in the 1980’s [1]. T7 Polymerase is also non-natively expressed in E. coli. Our team aimed to take advantage of this fact to decrease leaky expression, as explained in the figure below. If T7 polymerase is expressed inducibly under the control of a LacI promoter, and the protein of interest is transcribed under the control of an inducible T7 promoter, you are unlikely to get high amounts of “leaky” production of your protein of interest. This is because it would require two simultaneous “leaks” at two inducible promoters, rather than just one leak. Clearly, T7 Polymerase is a useful promoter to add to the synthetic biologist’s repertoire. Therefore, we were surprised to see there were no inducible T7 Promoters that were well-documented and verified to function in the registry. BBa_R0184,BBa_R0185,BBa_R0186, and BBa_R0187 were all supposed to be inducible T7 promoters, yet they had no functional verification. We sought to improve these parts by developing our own T7 promoter that could be induced.
Results
We designed our T7 promoter so that it could be induced by IPTG. Therefore, we simply ligated the Lac Operator sequence to the popular BBa_I712074 constitutive T7 promoter. Sequencing results (accessible by download and linked on this page) showed that we had successfully attached the T7-LacO promoter to our recombinase protein expression units, creating inducible expression generators (BBa_K2406080, BBa_K2406081, BBa_K2406082, BBa_K2406083, BBa_K2406084). We assayed the inducible activity of each recombinase generator. The image below shows the activity of Dre Recombinase protein. Focus on bars indicating Rox-Rox constructs (BBa_K2406051 part has more information on how this assay works), which were tested with and without IPTG. Essentially, RFP would be expressed and give off fluorescence when Dre recombinase expression was induced. Clearly, there is a significant difference between induced and non-induced control, demonstrating that our T7-LacO promoter works as would be desired.
Discussion
There was some leakiness observed by the T7-LacO promoter. Comparing un-induced Rox-Rox construct to other controls that did not give RFP output with or without Dre recombinase, there is clearly significantly more RFP fluorescence in the un-induced Rox-Rox construct. This indicates some Dre recombinase being produced despite no induction occurring. As explained in the introduction, we attempted to decrease leakiness by inducing T7 LacO expression as well. Leakiness could have perhaps been further reduced by using a straing carrying pLysS plasmid, which would express lysosyme to degrade any T7 polymerase produced through leakiness. Furthermore, other teams could attempt to alter the sequence of our promoter, perhaps by decreasing affinity for T7 RNA polymerase, in an attempt to decrease the intrinsic leakiness of this promoter. However, we have clearly produced an inducible T7 promoter that functions, therefore improving parts BBa_R0184, BBa_R0185, BBa_R0186, and BBa_R0187, all of which had slightly different sequences and no evidence of functioning.
References
Milligan, J.F., Groebe, D.R., Witherell, G.W., and Uhlenbeck, O.C. 1987. “Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates”. Nucleic Acids Research 15 (21): 8783-8798
Sequences
Find sequences of all promoter-regulated generators and measurement constructs here: Media:File:Sequencing Results Edinburgh UG.zip
Team Tec-Monterrey characterization
While we were working in the expression of our chimeric proteins we realized that the promoter has an important leakage.
We first noticed visually by noticeable fluorescence on non-induced bacteria.
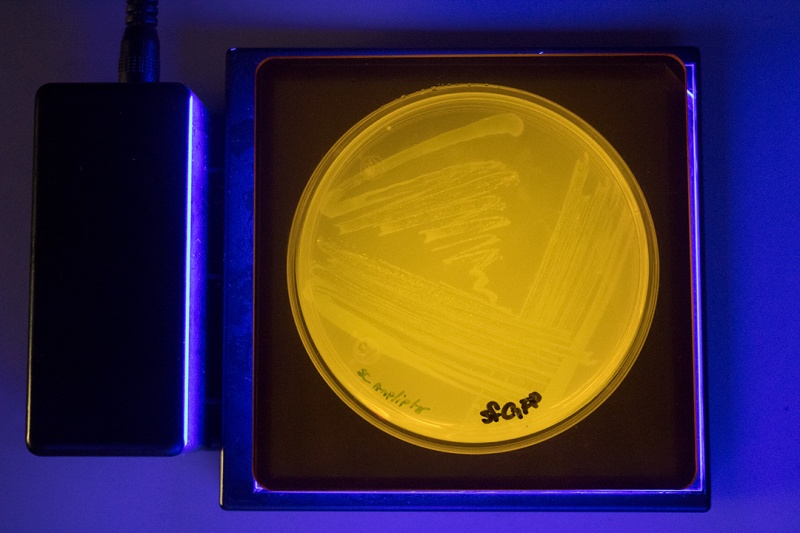
Figure 1. 200µM IPTG induced sfGFP transformed bacteria (note that it flouresces less than the non-induced one).

Figure 2. non-induced E.coli.
We confirmed the leakage with a fluorescence measurement, where bacteria were growth at 30ºC for 3 hrs. Then 100µl of the grown bacteria were induced with IPTG at 0, 40, 200 and 400 mMm microtiter florescent measures were taken every half an hour for a period of 3 hours. Absorption and emission values were 395/509 nm.
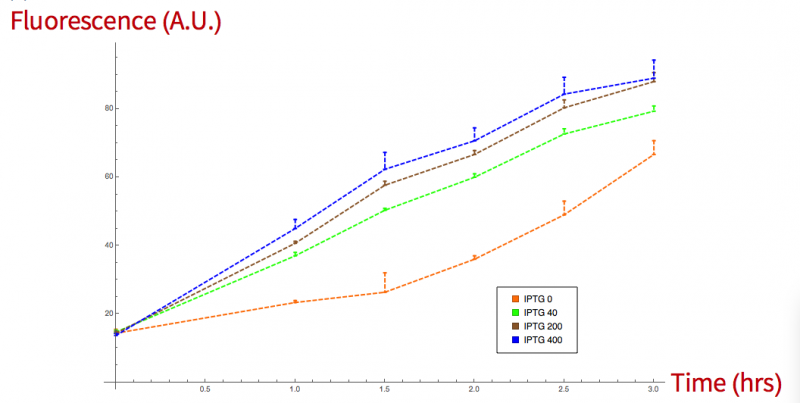
Figure 3. sfGFP grpah, fluorescence vs time induced at different concentrations.

Figure 4. sial+sfGFP grpah, fluorescence vs time induced at different concentrations.
NOTE: We suggest that the lower fluorescence values in the induction of the sfGFP+sialidase than the sfGFP itself are due to the different sizes of the chimeric proteins that could difficult its secretion.
Improvement By BJU_China iGEM2021
Description
T7 promoter is commonly used in iGEM projects, BBa_K2406020 designed by iGEM17_Edinburgh_UG was an inducile T7-LacO promoter for gene expression. In our project, we also used T7-LacO promoter to control the expression of halogenase. However, we found high basic leakage of this promoter, which affected the results of our experiments, so we improved this promoter in our project. It is recognized that an excellent promoter should possess exceptional switch ratio: it should have lower background leakage in the "off" state and have obvious expression in the "on" state. In order to reduce the leakage and to ensure the repressor can bind better to the LacO site, we added another LacO site in the T7-LacO promoter as the figure below shows:
Gene Circuit and Test Method
In the experiment, LacI was designed as a repressor and IPTG as an inducer to control the state of T7 promoter. The gene circuit diagram is shown in Fig. 2. At the same time, we use sfGFP as reporter gene, so that the fluorescence value can be used to quantitatified gene expression level. The two gene circuits were constructed on pET28b vector and transformed to E.coli BL21(DE3).
Single colonies of two bacteria (control, improved) were inoculated into LB medium, and cultivated vernight at 37° as seed culture. The seed culture was inoculated into M9 medium as 1:100, and IPTG were added at 0h respectively. The concentration of IPTG was 0, 10, 20, 50, 100, 200, 500, 1000 μM, and three parallel samples for each concentration. 200 μl of each sample were taken to measure the fluorescence after 6 hours of induction.
Results and Discussion
From the results shown in Fig.3,we can see that the improved T7-LacO promoter is much better than the control group in terms of lower basic leakage or higher expression status. At the zero point,that is without inducer, the leakage of improved promoter is more than ten times lower than the control group. While when enough inducer exist, that is, when the gene circuit is fully turned on, the maximum expression of the optimized promoter is not suppressed, and even slightly higher than the control group. Furthermore, when comparing the switch ratios of the two inducible promoters, the ratio of the control group was only about 12, while the switch ratio of improved promoter could reach 147, which also shows that our modification of this part is effective.
In the above experiments and results, we optimized the characterization performance of the T7-LacO promoter by adding an extra LacO site. This may be because in the lactose operon model, the LacI repressor is a tetramer that binds to the LacO site to control the state of inducible promoter. The function of LacO involves various factors such as DNA bending and interaction between protein and DNA. The specific influence mechanism of the amount of lacO on the promoter needs to be further studied.
Team Uni-Hamburg 2022 characterization
We wanted to use the T7 promoter to get an inducible control for different levels of fluorescence. We used the promoter together with BBa_E0034, BBa_E0040 and BBa_B0015 to build the inducible GFP unit. We then assembled it into the pSB1K3 backbone using goldengate assembly and transformed it into E.coli DH5alpha.
At this step we noticed high fluorescence even in the uninduced state.

Figure 1. Saving plate after colony PCR. Uninduced colonies containing the plasmid with the inducible GFP are clearly fluorescent while the plasmids not containing any GFP stay dark.
We further explored the leakage using a Tecan platereader with excitation at 485nm and emission at 515nm. The bacteria where cultured in 2 ml LB medium with kanamycin at 37°C for 6 hrs and then 250µl taken into a 96 well plate. We measured a serial 1:5 dilution. While cultures not containing the inducible GFP plasmid showed no fluorescence above background we could clearly observe fluorescence of the uninduced E.coli with the GFP plasmid. At a dilution of 1:25, even the GFP plasmids are not visible anymore as there are nearly no bacteria left.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]






