Difference between revisions of "Part:BBa K1980007"
Liu Jiankai (Talk | contribs) (→iGEM2022_Nanjing-China Experiment) |
|||
| (24 intermediate revisions by 4 users not shown) | |||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K1980007 short</partinfo> | <partinfo>BBa_K1980007 short</partinfo> | ||
| + | ==Description== | ||
| + | <html><p> | ||
| + | This part consist of the promoter pCusC (<a href="https://parts.igem.org/Part:BBa_K1980004">BBa_K1980004</a>), a copper responsive promoter activated by the CusS/R two component system, with a downstream RFP variant (mKate). </p></html> | ||
| − | + | We found a version of the pCusC promoter in the registry here: <html>(<a href="https://parts.igem.org/Part:BBa_I760005">BBa_I760005</a>)</html>. This part had 11 uses but had no expression data associated with any of these. As the part is only 16 nucleotides long, we believe it is far too small and missing important binding sites for both CusR and RNA polymerase. The version used in this part is extended to include these sites. | |
| − | + | ||
| − | We found a version of the pCusC promoter in the registry here: (https://parts.igem.org/Part:BBa_I760005). This part had 11 uses but had no expression data associated with any of these. As the part is only 16 nucleotides long we believe it is far too small and missing important binding sites for both CusR and RNA polymerase. | + | |
<!-- --> | <!-- --> | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
| Line 11: | Line 12: | ||
==Usage and Biology== | ==Usage and Biology== | ||
| + | Our project was to investigate a probiotic treatment for the copper-accumulation disorder: Wilson's disease. This required a system able to detect dietary copper, ideally in the range over which copper concentration changes after a meal (around 5-10μM). | ||
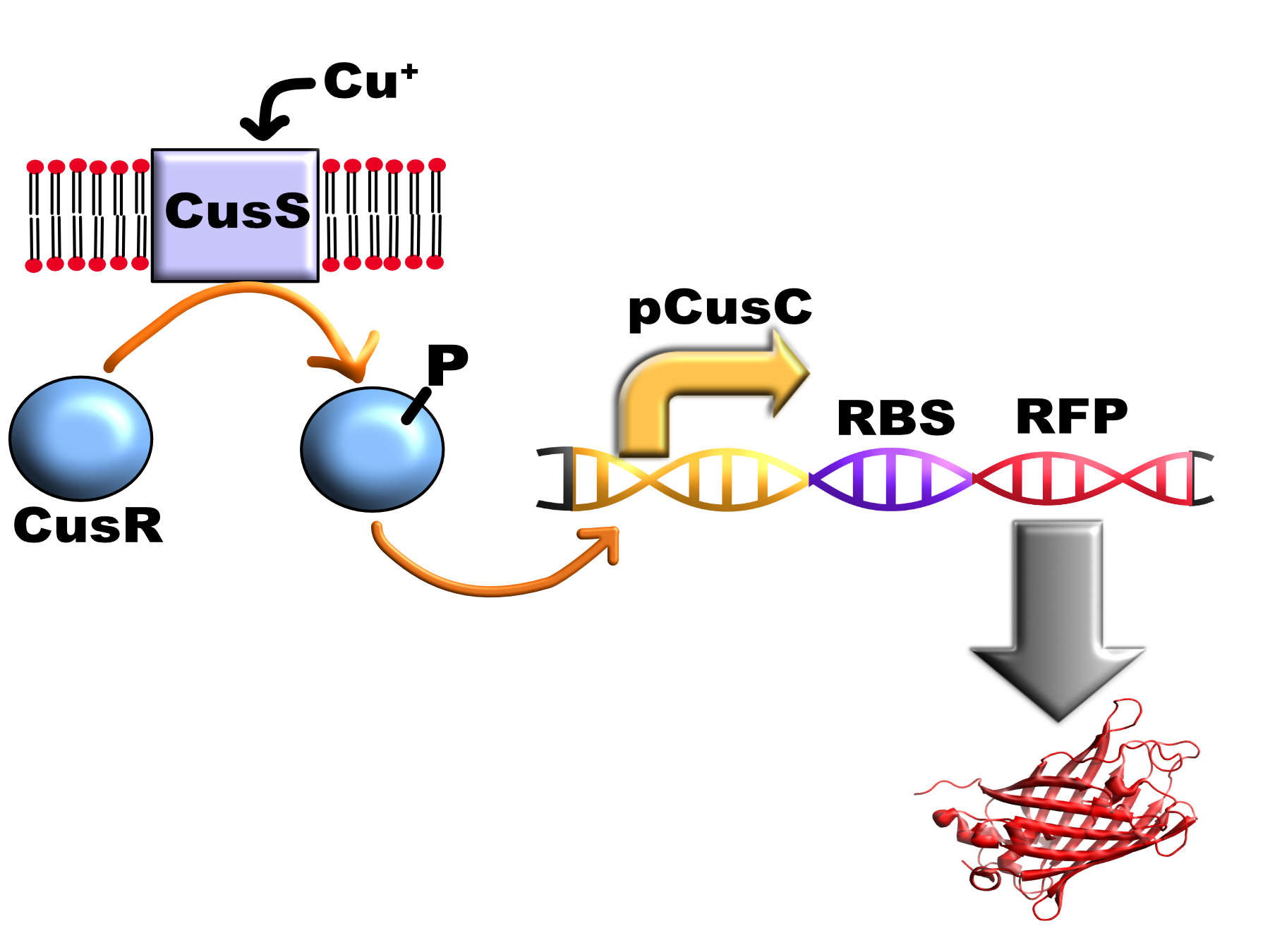
| − | <p> | + | <p>The system that <i>E. coli</i> uses to respond to external copper is the CusS/CusR two-component system. This consists of the transmembrane histidine kinase enzyme CusS in the bacterial cytoplasmic membrane and a cytoplasmic response regulator, CusR. |
When CusS binds periplasmic copper it transfers a phosphate group from ATP to CusR aspartate residue 51 via CusS histidine residue 271. Phosphorylated CusR can bind to DNA inverted repeat CusR boxes (AAAATGACAANNTTGTCATTTT) found in the pCusC promoter and activate gene expression. | When CusS binds periplasmic copper it transfers a phosphate group from ATP to CusR aspartate residue 51 via CusS histidine residue 271. Phosphorylated CusR can bind to DNA inverted repeat CusR boxes (AAAATGACAANNTTGTCATTTT) found in the pCusC promoter and activate gene expression. | ||
| Line 49: | Line 51: | ||
===Flow cytometry=== | ===Flow cytometry=== | ||
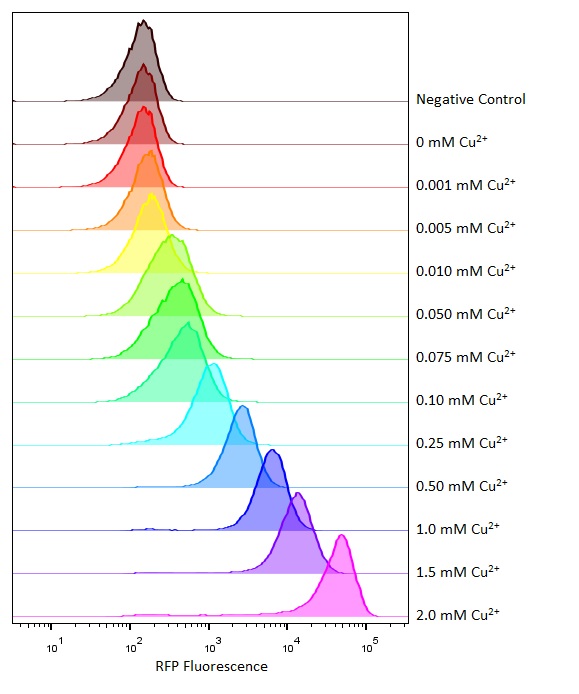
| − | [[Image:T--Oxford--Sam-pcusC-flowcyto.jpg|400px|thumb|right| | + | [[Image:T--Oxford--Sam-pcusC-flowcyto.jpg|400px|thumb|right|The population distribution of this part at different copper concentrations as measured by flow cytometry]] |
<p> | <p> | ||
Flow cytometry is a technique whereby cells are passed individually into the path of a beam of light. The frequency range of incident light can be adjusted to allow excitation of specific fluorophores in the sample of cells. Downstream detectors can measure fluorescent emission, and this data can be used to quantify the amount of fluorophore in each cell.</p> | Flow cytometry is a technique whereby cells are passed individually into the path of a beam of light. The frequency range of incident light can be adjusted to allow excitation of specific fluorophores in the sample of cells. Downstream detectors can measure fluorescent emission, and this data can be used to quantify the amount of fluorophore in each cell.</p> | ||
| Line 88: | Line 90: | ||
</p> | </p> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| Line 103: | Line 101: | ||
| + | <!--from this--> | ||
| + | <!--from this--> | ||
| + | ==iGEM2022_Nanjing-China Experiment== | ||
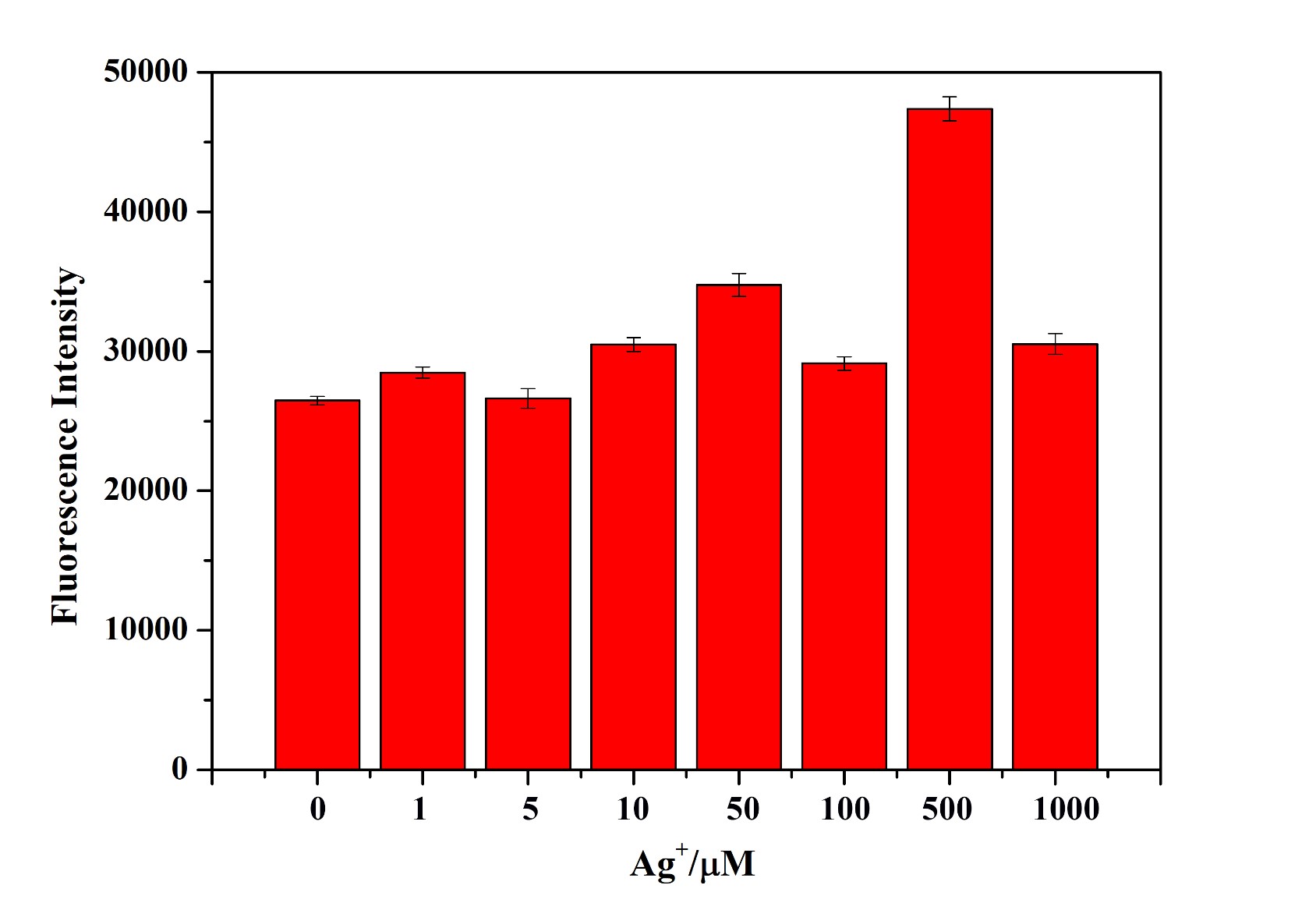
| + | [[Image:Nanjing-China-silver-CusS-RFP.jpg|400px|thumb|right|Fluorescence detection of transformed E.coli. at different concentrations of silver ions]] | ||
| + | <div> | ||
| + | <b>Group: Nanjing-China 2022</b> | ||
| + | <br> | ||
| + | <b>Author: Jiankai Liu</b> | ||
| + | <br><br> | ||
| − | === | + | <p> |
| + | We add new documentation to this existing Part, pCusC mKate2 (BBa_K1980007), and expand its applications in silver ions indicator through paper reading and our experiment results. | ||
| + | </p> | ||
| + | <p> | ||
| + | This part consists of the promoter pCusC (BBa_K1980004), a copper responsive promoter activated by the CusS/R two-component system, with a downstream RFP variant (mKate). According to the reference we found, the histidine kinase CusS of the CusR/S two-component system functions as an Ag(I)/Cu(I)-responsive sensor kinase, which is not unexpected due to the similar chemical properties of silver and copper. These findings suggest a model for activation of the histidine kinase through metal binding events in the periplasmic sensor domain. And the histidine kinase of CusS is the key to triggering downstream reactions including RFP expression. | ||
| + | </p> | ||
| + | <p> | ||
| + | We also carried out the experimental study including fluorescence detection of edited E. coli at different concentrations of silver ions. The experimental results support our speculation to some extent and further experiments are needed to confirm that. | ||
| + | </p> | ||
| + | |||
| + | <!--to here--> | ||
| + | |||
| + | ===Functional Parameters=== | ||
| + | |||
| + | <partinfo>BBa_K1980004 parameters</partinfo> | ||
| + | |||
| + | ==References== | ||
<p>(1) Yamamoto K, Ishihama A. (2005) “Transcriptional response of Escherichia coli to external copper.” Mol Microbiol. 2005 Apr;56(1):215-27.</p> | <p>(1) Yamamoto K, Ishihama A. (2005) “Transcriptional response of Escherichia coli to external copper.” Mol Microbiol. 2005 Apr;56(1):215-27.</p> | ||
| + | <p>(2) Gudipaty S A, McEvoy M M. The histidine kinase CusS senses silver ions through direct binding by its sensor domain[J]. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 2014, 1844(9): 1656-1661. | ||
| + | </p> | ||
Latest revision as of 17:20, 10 October 2022
pCusC mKate2
Description
This part consist of the promoter pCusC (BBa_K1980004), a copper responsive promoter activated by the CusS/R two component system, with a downstream RFP variant (mKate).
We found a version of the pCusC promoter in the registry here: (BBa_I760005). This part had 11 uses but had no expression data associated with any of these. As the part is only 16 nucleotides long, we believe it is far too small and missing important binding sites for both CusR and RNA polymerase. The version used in this part is extended to include these sites. Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
Our project was to investigate a probiotic treatment for the copper-accumulation disorder: Wilson's disease. This required a system able to detect dietary copper, ideally in the range over which copper concentration changes after a meal (around 5-10μM).
The system that E. coli uses to respond to external copper is the CusS/CusR two-component system. This consists of the transmembrane histidine kinase enzyme CusS in the bacterial cytoplasmic membrane and a cytoplasmic response regulator, CusR. When CusS binds periplasmic copper it transfers a phosphate group from ATP to CusR aspartate residue 51 via CusS histidine residue 271. Phosphorylated CusR can bind to DNA inverted repeat CusR boxes (AAAATGACAANNTTGTCATTTT) found in the pCusC promoter and activate gene expression. In E. coli this box is present between the promoters for CusCFBA operon which encodes a multi-protein pump that exports cytoplasmic and periplasmic copper from the cell and the CusRS operon which encodes the two component system (therefore acting as a positive feedback loop in vivo)(1).
We used this part to test the pCusC promoter using the fluorescent protein mKate2 (a form of RFP) as a reporter gene.
Experience
We measured the fluorescence of cells containing this part after copper stimulation using three different techniques: a plate reader experiment, flow cytometry and fluorescence microscopy:
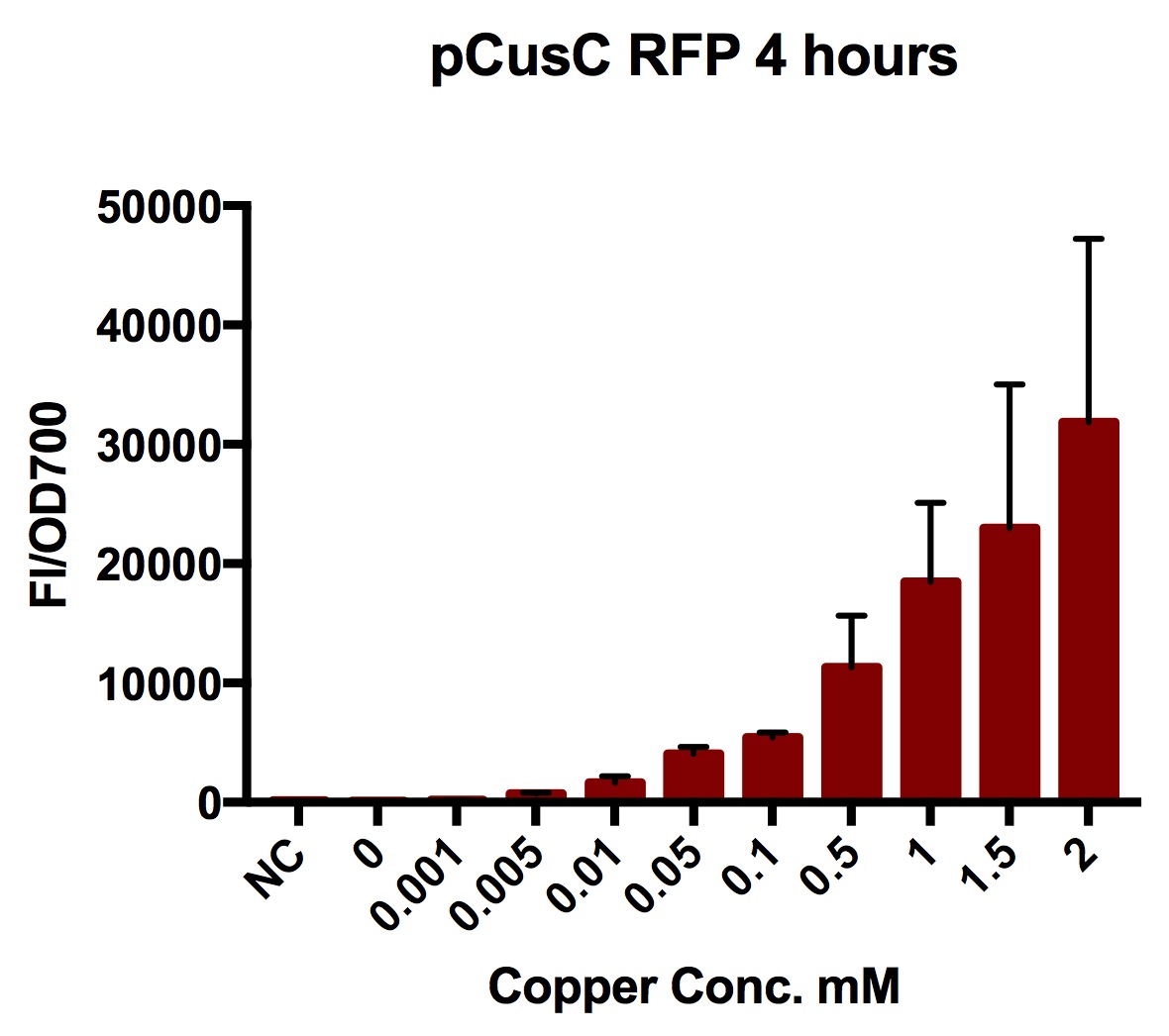
Plate Reader
We tested the pCusC promoter with the fluorescent protein mKate2 (a form of RFP excitation/emission maxima at 588 and 633 nm).
To account for the number of cells present at different copper concentrations and different times we measured the optical density (OD) as a proportional measure of the number of cells present. For this part we measured the optical density at 700nm because measuring OD600 would get interference from the fluorescent protein.
Plate reader experiments were prepared by picking individual colonies off stored plates into 5ml of LB with 1 in 1000 chloramphenicol and growing these overnight (at least 8 hours).
A range of copper concentrations were prepared from stock solutions. A large volume plate was then prepared with 10μl of copper solution, 10μl of overnight culture and 980μl of broth with antibiotic. This resulted in a 1 in 100 dilution of the copper solutions prepared.
This large-volume plate was then centrifuged to mix the solutions and then 200μl transferred to a small-volume plate with a clear lid and then placed in the plate reader.
Four biological repeats of this part at ten different copper concentrations were measured as well as four repeats of a MG1655 negative control.
The plate reader measured the fluorescence and OD every ten minutes for at least 12 hours, shaking between measurements.
Flow cytometry
Flow cytometry is a technique whereby cells are passed individually into the path of a beam of light. The frequency range of incident light can be adjusted to allow excitation of specific fluorophores in the sample of cells. Downstream detectors can measure fluorescent emission, and this data can be used to quantify the amount of fluorophore in each cell.
To ensure comparability of experiments, all cells were grown for 3-4hrs (until entrance into the exponential growth phase) at 37°C and 225rpm shaking in the presence of the inducer before measurement. This allowed adequate time for activation of expression by the promotor systems. The negative control used in all cases were MG1655 bacteria containing an empty shipping plasmid. As these did not contain any fluorescent molecules, this population could be used to set the negative “gate” (i.e. the background fluorescence of the bacterial cells). Although the experiments were tedious as every sample had to be measured manually, the results were of remarkably high quality, were clearly interpretable, and fit very well with the other experimental data.
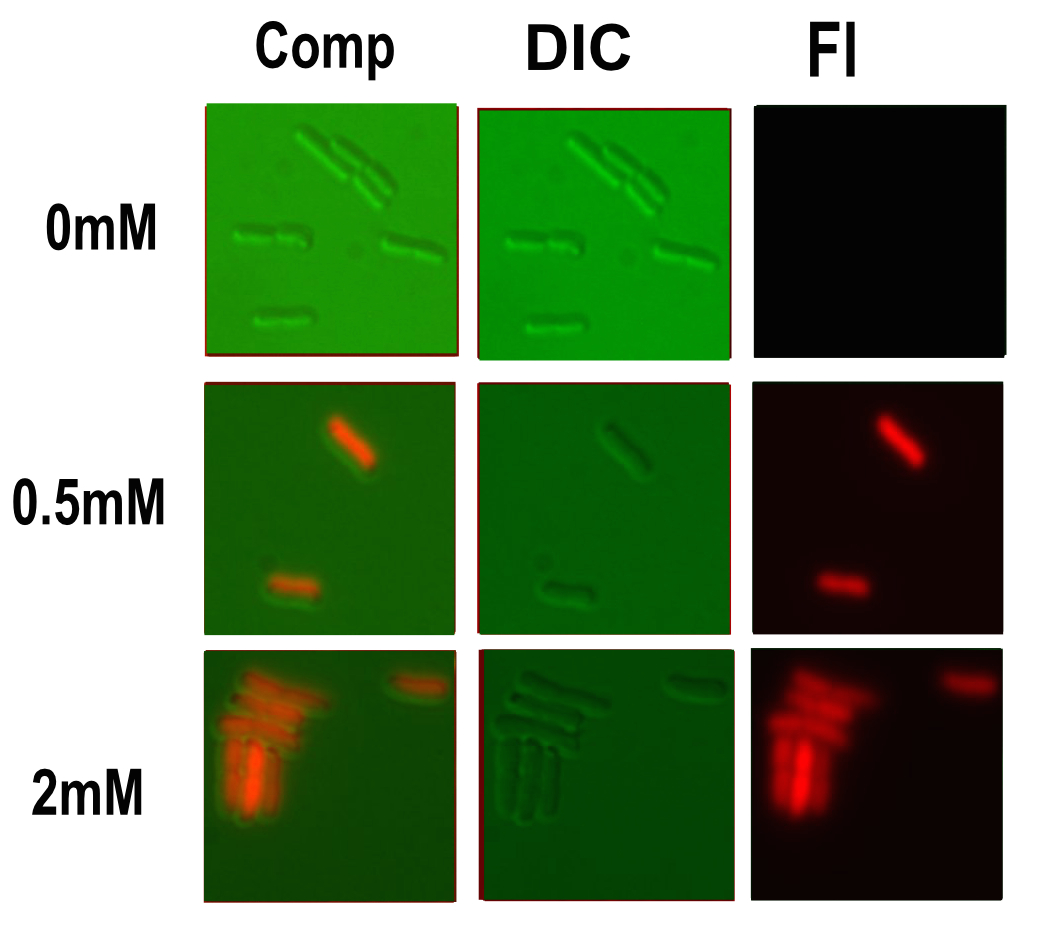
Microscopy
Microscopy was done in order to visually confirm the plate reader and flow cytometer experiments.
The experiment started with 5ml overnight cultures containing the appropriate antibiotic. Then in the morning 100μl of each colony was pipetted into 5ml of fresh LB with antibiotic and a range of copper concentrations and grown till the OD reached 0.4-0.6.
A flask of 1% agarose made with MilliQ was melted. 200μl of this was placed on a slide between two coverslips, flattened to get a nice smooth surface where the bacteria are immobile. 20μl of the culture are then added. The slide was then be viewed under a fluorescence microscope.
After finding the correct focal plane the slide was moved to find as many cells as possible to image. After focusing again an image of the DIC and fluorescence channels was obtained.
iGEM2022_Nanjing-China Experiment
Group: Nanjing-China 2022
Author: Jiankai Liu
We add new documentation to this existing Part, pCusC mKate2 (BBa_K1980007), and expand its applications in silver ions indicator through paper reading and our experiment results.
This part consists of the promoter pCusC (BBa_K1980004), a copper responsive promoter activated by the CusS/R two-component system, with a downstream RFP variant (mKate). According to the reference we found, the histidine kinase CusS of the CusR/S two-component system functions as an Ag(I)/Cu(I)-responsive sensor kinase, which is not unexpected due to the similar chemical properties of silver and copper. These findings suggest a model for activation of the histidine kinase through metal binding events in the periplasmic sensor domain. And the histidine kinase of CusS is the key to triggering downstream reactions including RFP expression.
We also carried out the experimental study including fluorescence detection of edited E. coli at different concentrations of silver ions. The experimental results support our speculation to some extent and further experiments are needed to confirm that.
Functional Parameters
| chassis | E. coli |
| positive_regulators | CusR |
References
(1) Yamamoto K, Ishihama A. (2005) “Transcriptional response of Escherichia coli to external copper.” Mol Microbiol. 2005 Apr;56(1):215-27.
(2) Gudipaty S A, McEvoy M M. The histidine kinase CusS senses silver ions through direct binding by its sensor domain[J]. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 2014, 1844(9): 1656-1661.