Difference between revisions of "Part:BBa K2110105"
(→Results) |
|||
| (15 intermediate revisions by 2 users not shown) | |||
| Line 15: | Line 15: | ||
<h2>Rational Design</h2> | <h2>Rational Design</h2> | ||
<h3>Serine-based Catalytic Triad Mechanism & 3D Model Simulation</h3> | <h3>Serine-based Catalytic Triad Mechanism & 3D Model Simulation</h3> | ||
| − | Since PETase was found to contain a <b>GWSMG</b> motif in accordance with the <b>GXGXG</b> motif<sup>[2]</sup>, which is characteristic and highly conserved in α/β hydrolase fold family, we simulated a best fit model for PETase by SWISSMODEL with a template as Thc_Cut2. As expected, the homology model of PETase displays a canonical α/β hydrolase fold with a Ser<sup> | + | Since PETase was found to contain a <b>GWSMG</b> motif in accordance with the <b>GXGXG</b> motif<sup>[2]</sup>, which is characteristic and highly conserved in α/β hydrolase fold family, we simulated a best fit model for PETase by SWISSMODEL with a template as Thc_Cut2. As expected, the homology model of PETase displays a canonical α/β hydrolase fold with a Ser<sup>160</sup>-His<sup>237</sup>-Asp<sup>206</sup> catalytic triad and a preformed oxyanion hole (Fig.1), suggesting a classic serine hydrolase mechanism. |
| − | + | <center><br/>https://static.igem.org/mediawiki/2016/5/57/Fig.1.PNG<br/></center> | |
| + | <center>Fig.1 Simulated 3D structure for PETase</center> | ||
| + | <br/> | ||
<h3>Mutation Design</h3> | <h3>Mutation Design</h3> | ||
| − | Based on the simulated 3D structure, we found the 208th | + | Based on the simulated 3D structure, we found the 208th amino acid residue isoleucine situates right over the catalytic triad(Fig.2). It is apperant that the interaction of catalytic triad with substrate would be inhibited due to the space oppcupied by 208th isoleucine. So we decided to substitue 208th isoleucine with another smaller amino acid. |
| − | <br/><br/> | + | <br/> |
| + | <center>https://static.igem.org/mediawiki/parts/3/33/10192.png</center> | ||
| + | <br/> | ||
However, as the isoleucine is the most hydrophobic amino acid, it takes risk to substitue it with another one, as it may lead to the hydrophobic amino acid decreasing. On balance, we chose valine as substitution, for its hydrophobicity is quite close to isoleucine, and the volume of valine is much smaller than that of isoleucine. | However, as the isoleucine is the most hydrophobic amino acid, it takes risk to substitue it with another one, as it may lead to the hydrophobic amino acid decreasing. On balance, we chose valine as substitution, for its hydrophobicity is quite close to isoleucine, and the volume of valine is much smaller than that of isoleucine. | ||
<br/><br/> | <br/><br/> | ||
| − | The 3D structure of mutant I208V is as Fig.3. Comparing to wild-type PETase(Fig.2), the mutant I208V exposes the catalytic triad much more widely. And the assay shows the stratety worked well for improving PET hydrolysis activity. The results are as follows. | + | The 3D structure of mutant I208V is as Fig.3. Comparing to wild-type PETase(Fig.2), the mutant I208V exposes the catalytic triad much more widely. And the assay shows the stratety worked well for improving PET hydrolysis activity. |
| + | <center>https://static.igem.org/mediawiki/parts/a/a3/10193.png</center> | ||
| + | <br/>The results are as follows. | ||
| Line 37: | Line 43: | ||
Than , the plasmids pRset_CFP-1_PETase-I208V was put into the CFPS(Cell-Free Protein Synthesis) to synthesis the enzymes we expected. | Than , the plasmids pRset_CFP-1_PETase-I208V was put into the CFPS(Cell-Free Protein Synthesis) to synthesis the enzymes we expected. | ||
| − | + | <center> https://static.igem.org/mediawiki/2016/e/e6/T--Tianjin--part-I208Vpc.png</center> | |
<h3>Degradation data</h3> | <h3>Degradation data</h3> | ||
| Line 43: | Line 49: | ||
<li>We detect the absorption peak of culture medium after culturing with PET film added for 5d. The absorption degree-wavelength curve is as follows.</li> | <li>We detect the absorption peak of culture medium after culturing with PET film added for 5d. The absorption degree-wavelength curve is as follows.</li> | ||
| − | https://static.igem.org/mediawiki/parts/thumb/7/7a/T--Tianjin--partdataI208V.png/800px-T--Tianjin--partdataI208V.png | + | <center>https://static.igem.org/mediawiki/parts/thumb/7/7a/T--Tianjin--partdataI208V.png/800px-T--Tianjin--partdataI208V.png</center> |
<li>We used the relative fluorescence units to represent the expression level of enzymes. </li> | <li>We used the relative fluorescence units to represent the expression level of enzymes. </li> | ||
| − | + | <center> https://static.igem.org/mediawiki/2016/1/1d/T--Tianjin--part-I208Vex.png</center> | |
<li>Finally we got the relative activities of enzymes. </li> | <li>Finally we got the relative activities of enzymes. </li> | ||
| − | + | <center> https://static.igem.org/mediawiki/2016/2/2f/T--Tianjin--part-I208Vbar.png</center> | |
| + | |||
| + | <br/><br/>So our result shows the rational design actually worked well and the mutant I208V shows a relatively improved hyfrolysis activity comparing to wild-type. Though as we can see in Fig.3, the substitution of isoleucine with valine is actually a very small change with a methyl on the 208<sup>th</sup> amino residue. However it leads to a two-fold improvement of enzyme activity, revealing that as we expected the 208<sup>th</sup> is a crucial site and the space adjacent to active center matters a lot. | ||
<h2>Reference</h2> | <h2>Reference</h2> | ||
| − | [1]Shaorong Chong. Overview of Cell-Free Protein Synthesis: Historic Landmarks, Commercial Systems, and Expanding Applications. Current Protocols in Molecular Biology 16.30.1-16.30.11, October 2014 DOI: 10.1002/0471142727.mb1630s108 | + | [1]Shaorong Chong. Overview of Cell-Free Protein Synthesis: Historic Landmarks, Commercial Systems, and Expanding Applications. Current Protocols in Molecular Biology 16.30.1-16.30.11, October 2014 DOI: 10.1002/0471142727.mb1630s108<br/><br/> |
| + | [2] Chen S, Tong X, Woodard R W, et al. Identification and characterization of bacterial cutinase[J]. Journal of Biological Chemistry, 2008, 283(38): 25854-25862. | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Latest revision as of 12:03, 20 October 2016
PETase site-directed mutant I208V
This is one of the site-directed mutants of the PETase we designed, we change the 208th amino acid isoleucine(I) with valine(V). The module is the Saccharomyces cerevisiae codon optimization version of PETase gene. We used site-directed mutagenesis PCR to obtain this mutant.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 79
Illegal BglII site found at 289 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Results
Rational Design
Serine-based Catalytic Triad Mechanism & 3D Model Simulation
Since PETase was found to contain a GWSMG motif in accordance with the GXGXG motif[2], which is characteristic and highly conserved in α/β hydrolase fold family, we simulated a best fit model for PETase by SWISSMODEL with a template as Thc_Cut2. As expected, the homology model of PETase displays a canonical α/β hydrolase fold with a Ser160-His237-Asp206 catalytic triad and a preformed oxyanion hole (Fig.1), suggesting a classic serine hydrolase mechanism.

Mutation Design
Based on the simulated 3D structure, we found the 208th amino acid residue isoleucine situates right over the catalytic triad(Fig.2). It is apperant that the interaction of catalytic triad with substrate would be inhibited due to the space oppcupied by 208th isoleucine. So we decided to substitue 208th isoleucine with another smaller amino acid.

However, as the isoleucine is the most hydrophobic amino acid, it takes risk to substitue it with another one, as it may lead to the hydrophobic amino acid decreasing. On balance, we chose valine as substitution, for its hydrophobicity is quite close to isoleucine, and the volume of valine is much smaller than that of isoleucine.
The 3D structure of mutant I208V is as Fig.3. Comparing to wild-type PETase(Fig.2), the mutant I208V exposes the catalytic triad much more widely. And the assay shows the stratety worked well for improving PET hydrolysis activity.

The results are as follows.
Assay system—CFPS system
CFPS system
Cell-free protein synthesis(CFPS) is a widely used method in molecular biology. Production of proteins using cell-free protein synthesis usually takes a few hours, in contrast to production of proteins in cells, which typically takes days to weeks. In fact, even first-time users can often obtain newly synthesized proteins in one day using a commercial system.
The diversity of the cell-free systems allows in vitro synthesis of a wide range of proteins for a variety of downstream applications, such as screeening of enzymes activities. In the post-genomic era, cell-free protein synthesis has rapidly become the preferred approach for high-throughput functional and structural studies of proteins and a versatile tool for in vitro protein evolution and synthetic biology[1].
Plasmid construction and expression in CFPS system
Ligaion of digested pRset_CFP-1, digested CFP gene and digested PETase(I208V) gene in accordance with the 1:5:5 molecular ratio. The newly constructed plasmid is called pRset_CFP-1_PETase-I208V. Than , the plasmids pRset_CFP-1_PETase-I208V was put into the CFPS(Cell-Free Protein Synthesis) to synthesis the enzymes we expected.

Degradation data
After the enzymes were expressed in the system successfully, we used the proteins we got to degrade PET and detected the degradation product, MHET, which has no other characteristic adsorption peak except at 260nm.

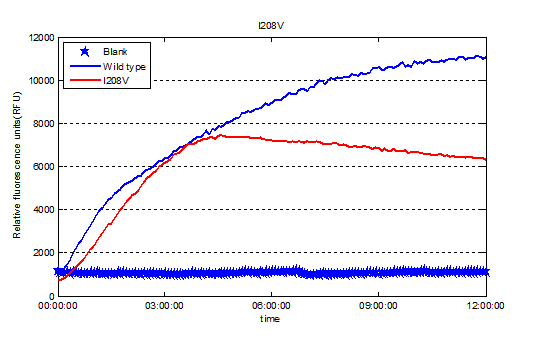

So our result shows the rational design actually worked well and the mutant I208V shows a relatively improved hyfrolysis activity comparing to wild-type. Though as we can see in Fig.3, the substitution of isoleucine with valine is actually a very small change with a methyl on the 208th amino residue. However it leads to a two-fold improvement of enzyme activity, revealing that as we expected the 208th is a crucial site and the space adjacent to active center matters a lot.
Reference
[1]Shaorong Chong. Overview of Cell-Free Protein Synthesis: Historic Landmarks, Commercial Systems, and Expanding Applications. Current Protocols in Molecular Biology 16.30.1-16.30.11, October 2014 DOI: 10.1002/0471142727.mb1630s108
[2] Chen S, Tong X, Woodard R W, et al. Identification and characterization of bacterial cutinase[J]. Journal of Biological Chemistry, 2008, 283(38): 25854-25862.
