Difference between revisions of "Part:BBa K2065004"
Tjvsonsbeek (Talk | contribs) (→Orthogonality of the scaffold) |
Tjvsonsbeek (Talk | contribs) (→Orthogonality of the scaffold) |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 17: | Line 17: | ||
<p> | <p> | ||
| − | The T14-3-3 – T14-3-3(W237R) | + | The T14-3-3 – T14-3-3(W237R) consists of a wildtype monomer and a monomer with mutation W237R, where the 237th amino acid is changed from tryptophan to arginine. This heterodimer is a scaffold protein and originates from the Tobacco plant. Protein-Protein Interactions (PPI) can be induced on this scaffold by linking proteins of choice to the CT52 protein, a protein with a known interaction with this scaffold protein. |
</p> | </p> | ||
<p> | <p> | ||
| Line 62: | Line 62: | ||
===Verification of the scaffold=== | ===Verification of the scaffold=== | ||
| − | To verify whether the scaffold shows the expected activity the activity of the scaffold at different concentrations was measured. | + | To verify whether the scaffold shows the expected activity the activity of the scaffold at different concentrations was measured. This is visible in figure 2. This gives the indication that T14-3-3(W237R)-T14-3-3 is not a functional scaffold. |
[[File:T--TU-Eindhoven--RelinsCom.png]] | [[File:T--TU-Eindhoven--RelinsCom.png]] | ||
| Line 83: | Line 83: | ||
===Orthogonality of the scaffold=== | ===Orthogonality of the scaffold=== | ||
| − | To check whether this heterodimeric scaffold protein is orthogonal, the following was done. The magnitude of the integrated luminescence for complementary CT52-split NanoLuc proteins ( CT52(S953K)-LargeBiT and CT52-SmallBit) was compared with three other situations. The first one consisted of two mutated CT52-split NanoLuc proteins (CT52(S953K)-LargeBiT and CT52(S953K)-SmallBit) instead of one wildtype and one mutated CT52-split nanoluc protein. The second one consisted of two wildtype CT52-split NanoLuc proteins (CT52-LargeBiT and CT52-SmallBit). The last one consisted of the two complementary CT52-split NanoLuc proteins without scaffold present. As a negative control substrate without scaffold protein of CT52-split NanoLuc proteins was used. | + | To check whether this heterodimeric scaffold protein is orthogonal, the following was done. The magnitude of the integrated luminescence for complementary CT52-split NanoLuc proteins ( CT52(S953K)-LargeBiT and CT52-SmallBit) was compared with three other situations. The first one consisted of two mutated CT52-split NanoLuc proteins (CT52(S953K)-LargeBiT and CT52(S953K)-SmallBit) instead of one wildtype and one mutated CT52-split nanoluc protein. The second one consisted of two wildtype CT52-split NanoLuc proteins (CT52-LargeBiT and CT52-SmallBit). The last one consisted of the two complementary CT52-split NanoLuc proteins without scaffold present. As a negative control substrate without scaffold protein of CT52-split NanoLuc proteins was used. This is visible in figure 4. For the conclusion about the effectivity of this mutation visit "http://2016.igem.org/Team:TU-Eindhoven/Results/Conclusion". |
| Line 89: | Line 89: | ||
<br> | <br> | ||
'' | '' | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Figure 1: T14-3-3 orthogonality measurement with 1 µM T14-3-3(W237R)-T14-3-3 concentration for bar 1-3, 0 µM T14-3-3(W237R)-T14-3-3 for bar 4 and 5. 50 µM fusicoccin for bar 1 -5. Mutant forms CT52-LargeBiT and CT52-SmallBiT are present in different combinations. Bar 1: CT52(S953K)-LargeBiT,CT52(wildtype)-SmallBiT. Bar 2: CT52(S953K)-LargeBiT,CT52(S953K)-SmallBiT. Bar 3: CT52(wildtype)-LargeBiT,CT52(wildtype)-SmallBiT. Bar 4: CT52(S953K)-LargeBiT,CT52(wildtype)-SmallBiT. Bar 5: No CT52-LargeBiT or CT52-SmallBiT present. The substrate used was 1250x diluted from stock. '' | Figure 1: T14-3-3 orthogonality measurement with 1 µM T14-3-3(W237R)-T14-3-3 concentration for bar 1-3, 0 µM T14-3-3(W237R)-T14-3-3 for bar 4 and 5. 50 µM fusicoccin for bar 1 -5. Mutant forms CT52-LargeBiT and CT52-SmallBiT are present in different combinations. Bar 1: CT52(S953K)-LargeBiT,CT52(wildtype)-SmallBiT. Bar 2: CT52(S953K)-LargeBiT,CT52(S953K)-SmallBiT. Bar 3: CT52(wildtype)-LargeBiT,CT52(wildtype)-SmallBiT. Bar 4: CT52(S953K)-LargeBiT,CT52(wildtype)-SmallBiT. Bar 5: No CT52-LargeBiT or CT52-SmallBiT present. The substrate used was 1250x diluted from stock. '' | ||
Latest revision as of 00:52, 19 October 2016
T14-3-3 (W237R) - T14-3-3
The T14-3-3 – T14-3-3(W237R) consists of a wildtype monomer and a monomer with mutation W237R, where the 237th amino acid is changed from tryptophan to arginine. This heterodimer is a scaffold protein and originates from the Tobacco plant. Protein-Protein Interactions (PPI) can be induced on this scaffold by linking proteins of choice to the CT52 protein, a protein with a known interaction with this scaffold protein.
The T14-3-3 (W237R) - T14-3-3 consist of a wildtype monomer and a monomer one mutation. The 237th amino acid is changed from tryptophan to arginine. This heterodimer is a scaffold protein and originates from the Tobacco plant. Protein-protein interactions can be induced on this scaffold by linking proteins of choice to the CT52 protein, a protein with a known interaction with this scaffold protein. The mutation on this protein is designed by iGEM TU Eindhoven. Specifications of this mutations can be found <a href="http://2016.igem.org/Team:TU-Eindhoven/Modeling/Rosetta_Results">here</a>
Biology of the construct
The 14-3-3 proteins are a family of proteins which are well preserved in evolution and are present in all eukaryotic cells. From the fact that there is a high diversity of interaction partners of the 14-3-3 protein, can be concluded that 14-3-3 contributes in many cell processes. [1] Examples of regulating and coordinating cell processes where 14-3-3 plays a role are cell cycle progression, apoptosis, metabolism, transcription, regulation of gene expression and DNA damage repair.
A variant of the 14-3-3 protein originates from the Nicotiana plumbaginifolia (Tobacco) plant and is called T14-3-3. [1] T14-3-3 proteins consist of two identical monomers, that dimerize to form a functional scaffold. Each monomer contains a bundle of nine antiparallel alfa-helices. Helices H3, H5, H7 and H9 form the “amphipathic ligand-binding groove”, in which other proteins can bind and interact with their co-protein. [2]
A protein that shows a high affinity for binding in the groove of T14-3-3 is called CT52. This proteins consist of the last 52 amino acids of the C-terminal region of the H+-ATPase. The N-terminus of CT52 is a free end, which can be used to link proteins to. The binding between CT52 and T14-3-3 is stabilized by the small molecule Fusicoccin. When Fusicoccin is present, the affinity of CT52 for T14-3-3 is increased in 30 fold with respect to the situation when Fusicoccin is not present [3]. The binding interaction with Fusicoccin is reversibel, this means CT52 will dissociate from the scaffold.
Gene Design and Usage
With help of the Rosetta software package, a molecular structure predictor that includes several algorithms for computational modelling and analysis of protein structures, new mutations for the T14-3-3 protein were found. A heterodimer is created to enable the assembly of two different protein-CT52 complexes. Orthogonality is introduced to ensure minimal interaction with the natural processes in that organism. These mutations have to be orthogonal with respect to the CT52 constructs. The mutation found by Rosetta is W237R, thus changing the 237th amino acid from a tryptophan to an arginine.
The T14-3-3 dimer now consist of a wildtype monomer and a monomer that contains the mutation W237R. The scaffold protein can therefore bind two different CT52-protein complexes. The statistics of this protein can be found below.
Sequence
The sequence of T14-3-3(W237R) - T14-3-3 has been verified by StarSeq. It contains the prefix and suffix with the correct restriction sites (EcoRI, XbaI, SpeI and PstI). Unfortunately this BioBrick contains 2 extra EcoRI restriction sites, it is important to be aware of this if you plan to use it. T14-3-3(W237R) - T14-3-3 is 1577 bp long.
Figure 1: Snapgene map of BBa_K2065004
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal EcoRI site found at 1234
Illegal EcoRI site found at 1389 - 12INCOMPATIBLE WITH RFC[12]Illegal EcoRI site found at 1234
Illegal EcoRI site found at 1389 - 21INCOMPATIBLE WITH RFC[21]Illegal EcoRI site found at 1234
Illegal EcoRI site found at 1389 - 23INCOMPATIBLE WITH RFC[23]Illegal EcoRI site found at 1234
Illegal EcoRI site found at 1389 - 25INCOMPATIBLE WITH RFC[25]Illegal EcoRI site found at 1234
Illegal EcoRI site found at 1389 - 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 937
Characterization
To verify the orthogonality and activity of T14-3-3 – T14-3-3(W237R) scaffold, in vitro assays have been performed with NanoLuc split luciferases linked to CT52. The NanoLuc reporter system consists of a small and a large bit, when those two are dimerized they are able to activate an luminogic substrate which emits luminscence at a wavelength of 460 nm. The small and large bit of this NanoLuc reporter system are called: SmallBiT and LargeBiT. The BioBricks of this NanoLuc reporter system linked to CT52 are <a href="">BBa_K2065000</a> and <a href="">BBa_K2065007</a>.
Verification of the scaffold
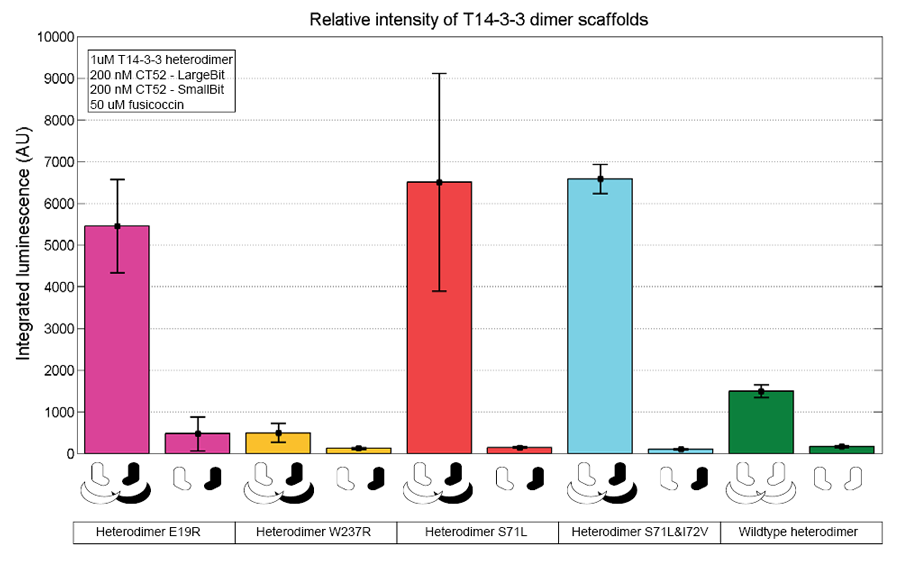
To verify whether the scaffold shows the expected activity the activity of the scaffold at different concentrations was measured. This is visible in figure 2. This gives the indication that T14-3-3(W237R)-T14-3-3 is not a functional scaffold.
Figure 2: T14-3-3 functionality measurement representing the relative intensities from each mutation set. The scaffold concentrations used are 1 µM. 50 µM fusicoccin was used for all measurements. CT52-LargeBiT and CT52-SmallBiT were used with a concentration of 200 nM, CT52-LargeBiT contained the mutation. The substrate used was 1250x diluted from stock.
To verify whether the scaffold shows the expected activity the activity of the scaffold at different concentrations was measured. Below a bar graph with the integrated bioluminescencescence over a timespan of 20 minutes. This reinforces that T14-3-3(W237R) - T14-3-3 is not a functional scaffold.
Figure 3: T14-3-3 functionality measurement with varying scaffold concentrations from 0 nM to 1 µM T14-3-3(W237R)-T14-3-3. 50 µM fusicoccin was used for all measurements. Mutant forms CT52 (S953K)-LargeBiT and CT52(wildtype)-SmallBiT were used with a concentration of 200 nM. The substrate used was 1250x diluted from stock.
Orthogonality of the scaffold
To check whether this heterodimeric scaffold protein is orthogonal, the following was done. The magnitude of the integrated luminescence for complementary CT52-split NanoLuc proteins ( CT52(S953K)-LargeBiT and CT52-SmallBit) was compared with three other situations. The first one consisted of two mutated CT52-split NanoLuc proteins (CT52(S953K)-LargeBiT and CT52(S953K)-SmallBit) instead of one wildtype and one mutated CT52-split nanoluc protein. The second one consisted of two wildtype CT52-split NanoLuc proteins (CT52-LargeBiT and CT52-SmallBit). The last one consisted of the two complementary CT52-split NanoLuc proteins without scaffold present. As a negative control substrate without scaffold protein of CT52-split NanoLuc proteins was used. This is visible in figure 4. For the conclusion about the effectivity of this mutation visit "http://2016.igem.org/Team:TU-Eindhoven/Results/Conclusion".

Figure 1: T14-3-3 orthogonality measurement with 1 µM T14-3-3(W237R)-T14-3-3 concentration for bar 1-3, 0 µM T14-3-3(W237R)-T14-3-3 for bar 4 and 5. 50 µM fusicoccin for bar 1 -5. Mutant forms CT52-LargeBiT and CT52-SmallBiT are present in different combinations. Bar 1: CT52(S953K)-LargeBiT,CT52(wildtype)-SmallBiT. Bar 2: CT52(S953K)-LargeBiT,CT52(S953K)-SmallBiT. Bar 3: CT52(wildtype)-LargeBiT,CT52(wildtype)-SmallBiT. Bar 4: CT52(S953K)-LargeBiT,CT52(wildtype)-SmallBiT. Bar 5: No CT52-LargeBiT or CT52-SmallBiT present. The substrate used was 1250x diluted from stock.
T14-3-3(W237R)-T14-3-3 statistics
| Protein Specifications | |||
|---|---|---|---|
| General Information | Number of amino acids | 514 | |
| Molecular weight | 56741.69 | ||
| Theoretical pi | 5.00 | ||
| Extinction coefficient | 55030 of 54780 | ||
| Formula | C2472H3946N682O809S18 | ||
| Total numbers of atons | 7927 | ||
| Amino Acid Composition | Amino Acid | Frequency | Percentage(%) |
| Ala(A) | 53 | 10.3 | |
| Arg(R | 29 | 5.6 | |
| Asn(N) | 22 | 4.3 | |
| Asp(D) | 22 | 4.3 | |
| Cys(C) | 4 | 0.8 | |
| Gln(Q) | 12 | 2.3 | |
| Glu(E) | 60 | 11.7 | |
| Gly(G) | 39 | 7.6 | |
| His(H) | 6 | 1.2 | |
| Ile(I) | 28 | 5.4 | |
| Leu(L) | 52 | 10.1 | |
| Lys(K) | 32 | 6.2 | |
| Met(M) | 14 | 2.7 | |
| Phe(F) | 12 | 2.3 | |
| Pro(P) | 10 | 1.9 | |
| Ser(S) | 50 | 9.7 | |
| Thr(T) | 24 | 4.7 | |
| Trp(W) | 3 | 0.6 | |
| Tyr(T) | 22 | 4.3 | |
| Val(V) | 20 | 3.9 | |
| Pyl(O) | 0 | 0.0 | |
| Sec(U) | 0 | 0.0 | |
References
[1] – Ottmann, C., Marco, S., Jaspert, N., Marcon, C., Schauer, N., Weyand, M., Vandermeeren, C., Duby, G., Boutry, M., Wittinghofer, A., Rigaud, J. and Oecking, C. (2007). Structure of a 14-3-3 Coordinated Hexamer of the Plant Plasma Membrane H+-ATPase by Combining X-Ray Crystallography and Electron Cryomicroscopy. Molecular Cell, 25(3), pp.427-440.
[2] - Obsil, T. and Obsilova, V. (2011). Structural basis of 14-3-3 protein functions. Seminars in Cell & Developmental Biology, 22(7), pp.663-672.
[3] - Milroy, L., Brunsveld, L., & Ottmann, C. (2013). Stabilization and Inhibition of Protein–Protein Interactions: The 14-3-3 Case Study. ACS Chem. Biol., 8(1), 27-35.