Difference between revisions of "Part:BBa K1886006"
| (2 intermediate revisions by one other user not shown) | |||
| Line 5: | Line 5: | ||
The red light sensor (Cph8) is a fusion protein which is consisted of a phytochrome Cph1 and a histidine kinase domain, Envz-OmpR, that includes a response regulator.Cph1 is the first member of the plant photoreceptor family that has been identified in bacteria. The functional expression of a phytochrome domain(Cph1) in E. coli requires the biosynthesis of the respective bilin chromophore PCB. EnvZ-OmpR, a dimeric osmosensor, is a multidomain transmembrane protein and one of the best characterized two-component histidine kinases from E.coli. | The red light sensor (Cph8) is a fusion protein which is consisted of a phytochrome Cph1 and a histidine kinase domain, Envz-OmpR, that includes a response regulator.Cph1 is the first member of the plant photoreceptor family that has been identified in bacteria. The functional expression of a phytochrome domain(Cph1) in E. coli requires the biosynthesis of the respective bilin chromophore PCB. EnvZ-OmpR, a dimeric osmosensor, is a multidomain transmembrane protein and one of the best characterized two-component histidine kinases from E.coli. | ||
| + | |||
With the biosynthesis of PCB, Cph1 serve as a red-light absorbing chromophore that is inactivated under red light and activated without red light. Upon changes of extracellular osmolarity, EnvZ specifically phosphorylates its cognate response regulator OmpR, which, in turn, regulates the PompC. | With the biosynthesis of PCB, Cph1 serve as a red-light absorbing chromophore that is inactivated under red light and activated without red light. Upon changes of extracellular osmolarity, EnvZ specifically phosphorylates its cognate response regulator OmpR, which, in turn, regulates the PompC. | ||
| + | |||
This circuit is composed of the constitutive promoter -- broken Ptet and membrane protein cph8. Under dark conditions, protein cph8 could transfer its phosphoryl group to intracellular protein ompR (ompR is native protein carried by E.coli), and this process could be inhibited by 650-nm light. | This circuit is composed of the constitutive promoter -- broken Ptet and membrane protein cph8. Under dark conditions, protein cph8 could transfer its phosphoryl group to intracellular protein ompR (ompR is native protein carried by E.coli), and this process could be inhibited by 650-nm light. | ||
| Line 22: | Line 24: | ||
<partinfo>BBa_K1886006 parameters</partinfo> | <partinfo>BBa_K1886006 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | <h2>'''Characterization'''</h2> | ||
| + | <h3> BACKGROUND </h3> | ||
| + | <h4>Overview</h4> | ||
| + | Registered Parts in our wiki is the subunit of the entire light-controlled system, each of which has no function. So I will introduce our light input part of our project after the combination of all the subunits here. | ||
| + | <br> | ||
| + | <h4>Maps of Plasmids</h4> | ||
| + | [[File:CcaSR-system.png|50%|thumb|left|Fig.1 CcaS/R system ]] | ||
| + | [[File:Cph8-OmpR-system.png|50%|thumb|left|Fig.2 Cph8/OmpR system ]] | ||
| + | <h4>Principle</h4> | ||
| + | <br> | ||
| + | <h4>a. Principle of CcaS/R system</h4> | ||
| + | The SK CcaS is produced in a green-absorbing ground state, termed Pg. Absorption of green light flips CcaS to a kinaseactive red-absorbing state (Pr) that phosphorylates the response regulator CcaR, which then binds to the cpcG2 promoter and activates transcription . Absorption of red light switches CcaS Pr back to Pg, which dephosphorylates phospho-CcaR,deactivating transcription. | ||
| + | <br> | ||
| + | [[File:CcaSR-system-principle.png|800px|thumb|center|Fig.3 CcaSR system principle ]] | ||
| + | <br> | ||
| + | <h4>b. Principle of Cph8/OmpR system</h4> | ||
| + | The chimaeric light receptor Cph8 contains the photoreceptor from Cph1 and the histidine kinase and response-regulator from EnvZ–OmpR conversion of haem to phycocyanobilin (PCB), which forms part of the photoreceptor. Red light drives the sensor to a state in which autophosphorylation is inhibited turning off gene expression. | ||
| + | <br> | ||
| + | The SK Cph8 is produced in a kinase-active Pr state that phosphorylates the response regulator OmpR, activating transcription from the ompC promoter. Red light switches Cph8 Pr to a far red–absorbing state (Pfr), which dephosphorylates phospho-OmpR, deactivating transcription | ||
| + | [[File:Cph8-OmpR-system-principle.png|800px|thumb|center|Fig.3.1 Cph8/OmpR system principle.1 ]] | ||
| + | [[File:Cph8-OmpR-system-principle-2.png|800px|thumb|center|Fig.3.2 Cph8/OmpR system principle.2 ]] | ||
| + | <br> | ||
| + | <br> | ||
| + | <h3>RESULTS</h3> | ||
| + | <br> | ||
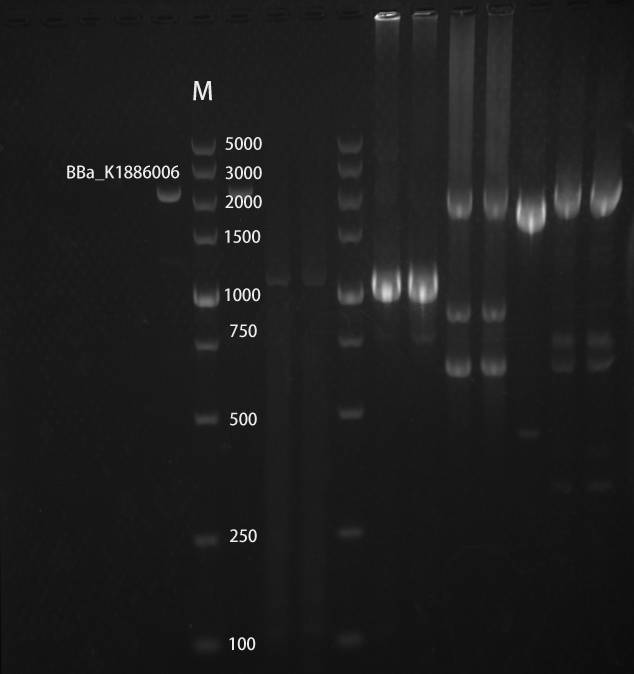
| + | <h4>Gel electrophoretic analysis</h4> | ||
| + | <br> | ||
| + | [[File:broken Ptet-cph8.jpg|800px|thumb|center|Fig.4 Gel electrophoretic analyses of PCR products ]] | ||
| + | |||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
Latest revision as of 02:17, 20 October 2016
broken Ptet-cph8
The red light sensor (Cph8) is a fusion protein which is consisted of a phytochrome Cph1 and a histidine kinase domain, Envz-OmpR, that includes a response regulator.Cph1 is the first member of the plant photoreceptor family that has been identified in bacteria. The functional expression of a phytochrome domain(Cph1) in E. coli requires the biosynthesis of the respective bilin chromophore PCB. EnvZ-OmpR, a dimeric osmosensor, is a multidomain transmembrane protein and one of the best characterized two-component histidine kinases from E.coli.
With the biosynthesis of PCB, Cph1 serve as a red-light absorbing chromophore that is inactivated under red light and activated without red light. Upon changes of extracellular osmolarity, EnvZ specifically phosphorylates its cognate response regulator OmpR, which, in turn, regulates the PompC.
This circuit is composed of the constitutive promoter -- broken Ptet and membrane protein cph8. Under dark conditions, protein cph8 could transfer its phosphoryl group to intracellular protein ompR (ompR is native protein carried by E.coli), and this process could be inhibited by 650-nm light.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 2332
Illegal XhoI site found at 439 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterization
BACKGROUND
Overview
Registered Parts in our wiki is the subunit of the entire light-controlled system, each of which has no function. So I will introduce our light input part of our project after the combination of all the subunits here.
Maps of Plasmids
Principle
a. Principle of CcaS/R system
The SK CcaS is produced in a green-absorbing ground state, termed Pg. Absorption of green light flips CcaS to a kinaseactive red-absorbing state (Pr) that phosphorylates the response regulator CcaR, which then binds to the cpcG2 promoter and activates transcription . Absorption of red light switches CcaS Pr back to Pg, which dephosphorylates phospho-CcaR,deactivating transcription.
b. Principle of Cph8/OmpR system
The chimaeric light receptor Cph8 contains the photoreceptor from Cph1 and the histidine kinase and response-regulator from EnvZ–OmpR conversion of haem to phycocyanobilin (PCB), which forms part of the photoreceptor. Red light drives the sensor to a state in which autophosphorylation is inhibited turning off gene expression.
The SK Cph8 is produced in a kinase-active Pr state that phosphorylates the response regulator OmpR, activating transcription from the ompC promoter. Red light switches Cph8 Pr to a far red–absorbing state (Pfr), which dephosphorylates phospho-OmpR, deactivating transcription
RESULTS
Gel electrophoretic analysis