Difference between revisions of "Part:BBa K319003"
(→Characterization) |
(→Characterization) |
||
| (34 intermediate revisions by 6 users not shown) | |||
| Line 75: | Line 75: | ||
| − | [[Image:T--Chalmers Gothenburg--glucose-acetate-relative.png|800px|thumb|center|Figure 1: Fluorescent levels of GFP under the control of the promoters pAQR1, pGLN1, pPCK1, PYK2 and pTEF1 in glucose and acetate conditions relative the levels of pTEF1. | + | [[Image:T--Chalmers Gothenburg--glucose-acetate-relative.png|800px|thumb|center|Figure 1: Fluorescent levels of GFP under the control of the promoters pAQR1, pGLN1, pPCK1, PYK2 and pTEF1 in glucose and acetate conditions relative the levels of pTEF1. Each sample was loaded into three different wells in the plate reader, and error bars are shown as confidence intervals with p = 0.05, using student's t-test.]] |
| + | All promoters except pPCK1 show higher expression relative pTEF1 at glucose conditions compared with acetate conditions, which is consistent with previous reports [1]. pPCK1 even has higher expression level than pTEF1, which means that pPCK1 could be preferred for overexpression when acetate is the only carbon source. | ||
| + | |||
| + | A more detailed version of the promoter study and how it's connected to our project can be found [http://2016.igem.org/Team:Chalmers_Gothenburg/Project/Promoter_study here]. | ||
*'''Uploads:''' <html><a href="https://static.igem.org/mediawiki/parts/6/65/T--Chalmers_Gothenburg-promoter-study-data.pdf">Promoter study data</a> | *'''Uploads:''' <html><a href="https://static.igem.org/mediawiki/parts/6/65/T--Chalmers_Gothenburg-promoter-study-data.pdf">Promoter study data</a> | ||
</html> | </html> | ||
| + | |||
| + | ==Contribution == | ||
| + | |||
| + | *'''Group:''' [http://2018.igem.org/Team:Peking Peking iGEM team 2018] | ||
| + | *'''Author:''' Chen Yuyang | ||
| + | *'''Summary:''' characterize the strength of this promoter and compare it with other promoters. | ||
| + | *'''Link to our biobrick: ''' https://parts.igem.org/Part:BBa_K2601037 | ||
| + | |||
| + | ===Characterization === | ||
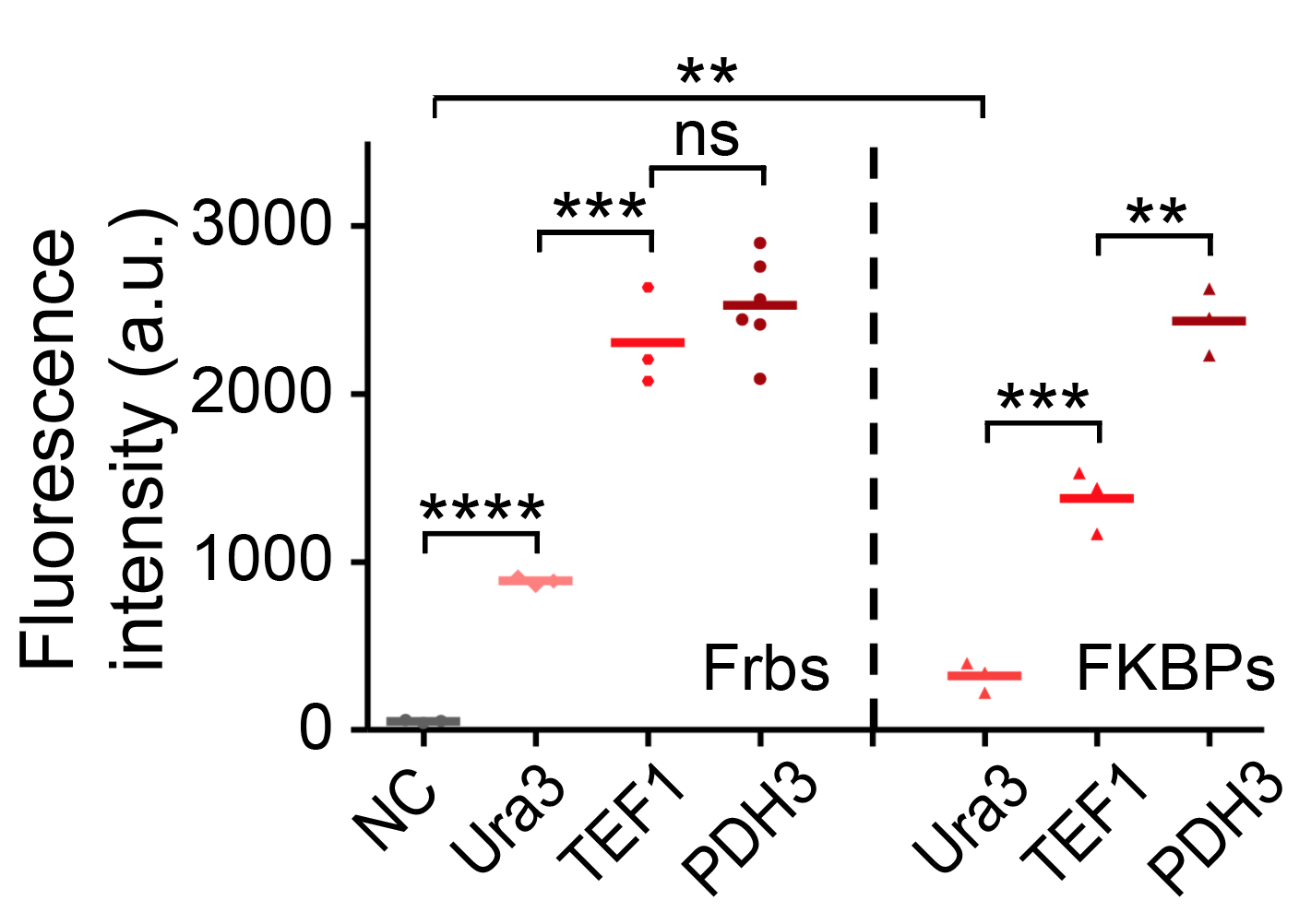
| + | We have tested the strength of TEF1 using flow cytometry and compared it with other promoters. The promoters are fused with Frb-HOTag6 and FKBP-HOTag3 respectively. | ||
| + | |||
| + | [[file:T--Peking--promoter-strength-new.png|500px|center|]] | ||
<!-- --> | <!-- --> | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K319003 SequenceAndFeatures</partinfo> | <partinfo>BBa_K319003 SequenceAndFeatures</partinfo> | ||
| − | |||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
| Line 90: | Line 104: | ||
<partinfo>BBa_K319003 parameters</partinfo> | <partinfo>BBa_K319003 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | ===References=== | ||
| + | [1] K. Weinhandl, M. Winkler, A. Glieder, and A. Camattari, “Carbon source dependent promoters in yeasts,” <i>Microbial Cell Factories</i>, vol. 13, no. 1, 2014 | ||
| + | |||
| + | ==Contribution == | ||
| + | |||
| + | *'''Group:''' [https://2020.igem.org/Team:SCU-China SCU-China iGEM team 2020] | ||
| + | *'''Author:''' Yilong Xu | ||
| + | *'''Summary:''' compare the strength of this promoter and pGAP. | ||
| + | *'''Link to our biobrick: ''' https://parts.igem.org/Part:BBa_K3544011 | ||
| + | |||
| + | ===Characterization === | ||
| + | To visualize the expression level of [https://parts.igem.org/Part:BBa_K3544011 pTEF1] and choose the appropriate promoter for NLS-Csy4 and function genes, we measure the fluorescence intensity, and the data was divided by OD. | ||
| + | |||
| + | [[image:T--SCU-China--scu-2020-results-15.png|600px|center|]] | ||
| + | Figure 1: Relative fluorescence intensity of two fluorescence proteins under two promoters. | ||
| + | |||
| + | The signal of [https://parts.igem.org/Part:BBa_K3544204 pGAP-CRISPR(Csy4)-DsRed-tADH1] is low, this is related to our plasmid design. We use double enzyme digestion and T4 ligation to construct pGAP-NLS-yeGFP-28nt-DsRed, the first step is the construction of pGAP-28nt-DsRed, which is called pGAP-R here. The secondary structure of 28nt may influence the expression level of DsRed. | ||
| + | |||
| + | In short, pTEF1 is stronger than pGAP (judged by the fluorescence of GFP and DsRed), so we use pTEF1 to express NLS-Csy4 for high cutting efficiency, and to design the construction of function genes additionally according to our modeling result. | ||
Latest revision as of 16:30, 27 October 2020
yeast TEF1 promoter
This is the full length TEF1 promoter including the distal and proximal portions and their regulatory elements.
Contribution
- Group: [http://2016.igem.org/Team:Chalmers_Gothenburg iGEM Team Chalmers Gothenburg 2016]
- Author: John Hellgren
- Summary: A promoter study to characterize this promoter and compare it against several others in two different conditions.
Characterization
A promoter study was performed to characterize this promoter. The reporter gene GFP was cloned into the replicative plasmid p416tef, downstream of the TEF1 promoter. By using a replicative plasmid instead of chromosomal integration, a higher copy number can be achieved, which will make sure that even weak promoters give a detectable signal. For the glucose conditions, the cells were grown as a preculture in SD -URA + 2 % glucose media overnight, diluted to OD600=0.3 in the same media and cultivated for 3 hours. The expression of GFP was measured in a 96-well plates (NUNC 96) in a BMG Labtech FLUOstar Omega plate reader with triplicate samples using the following setting: 20 flashes per well, excitation/emission wavelength at 485/520 nm and gain set to 800.
The cells were also grown in SD -URA + 0.5 % acetate to compare the expression levels when acetate was the only carbon source, which is connected to our coculture project. For the acetate experiment, the cells were grown as a preculture in SD -URA + 2 % glucose media overnight, washed and diluted to OD600=0.3 in SD -URA + 0.5 % acetate and cultivated for 24 hours before plate reader measurements. The longer cultivation time was due to slow growth with acetate as the carbon source. Furthermore, the reason for the longer cultivation time was to make sure that the GFP produced during the preculture in glucose was degraded.
The experiment was also done with the promoters pAQR1, pGLN1, pPCK1 and pTEF1 in the same way, and the results compared against each other. The raw data from the promoter study was normalized against OD600 of that sample, and the mean value of the negative control (cells with p416tef without GFP) was subtracted. The results are shown in Table 1.
PYK2 and pTEF1 for cells cultivated in SD -URA media + 2 % glucose or 0.5 % acetate (n=3).
| Promoter | Condition | |
|---|---|---|
| Glucose (fluorescent unit/OD600) |
Acetate (fluorescent unit/OD600) | |
| pAQR1 |
303 | 63 |
| pGLN1 |
862 | 426 |
| pPCK1 | 235 | 1721 |
| pPYK2 | 125 | 77 |
| pTEF1 | 1314 | 1399 |
In Figure 1 the results are normalized against the expression level of the pTEF1 promoter.

All promoters except pPCK1 show higher expression relative pTEF1 at glucose conditions compared with acetate conditions, which is consistent with previous reports [1]. pPCK1 even has higher expression level than pTEF1, which means that pPCK1 could be preferred for overexpression when acetate is the only carbon source.
A more detailed version of the promoter study and how it's connected to our project can be found [http://2016.igem.org/Team:Chalmers_Gothenburg/Project/Promoter_study here].
- Uploads: Promoter study data
Contribution
- Group: [http://2018.igem.org/Team:Peking Peking iGEM team 2018]
- Author: Chen Yuyang
- Summary: characterize the strength of this promoter and compare it with other promoters.
- Link to our biobrick: https://parts.igem.org/Part:BBa_K2601037
Characterization
We have tested the strength of TEF1 using flow cytometry and compared it with other promoters. The promoters are fused with Frb-HOTag6 and FKBP-HOTag3 respectively.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 205
References
[1] K. Weinhandl, M. Winkler, A. Glieder, and A. Camattari, “Carbon source dependent promoters in yeasts,” Microbial Cell Factories, vol. 13, no. 1, 2014
Contribution
- Group: SCU-China iGEM team 2020
- Author: Yilong Xu
- Summary: compare the strength of this promoter and pGAP.
- Link to our biobrick: https://parts.igem.org/Part:BBa_K3544011
Characterization
To visualize the expression level of pTEF1 and choose the appropriate promoter for NLS-Csy4 and function genes, we measure the fluorescence intensity, and the data was divided by OD.
Figure 1: Relative fluorescence intensity of two fluorescence proteins under two promoters.
The signal of pGAP-CRISPR(Csy4)-DsRed-tADH1 is low, this is related to our plasmid design. We use double enzyme digestion and T4 ligation to construct pGAP-NLS-yeGFP-28nt-DsRed, the first step is the construction of pGAP-28nt-DsRed, which is called pGAP-R here. The secondary structure of 28nt may influence the expression level of DsRed.
In short, pTEF1 is stronger than pGAP (judged by the fluorescence of GFP and DsRed), so we use pTEF1 to express NLS-Csy4 for high cutting efficiency, and to design the construction of function genes additionally according to our modeling result.