Difference between revisions of "Part:BBa K1893003:Experience"
(→Characterisation of BBa_K1893003) |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
| − | + | We used this part to engineer a bacterial communication system which was bidirectional, so that two different strains could grow together. Each strain in our system must have a distinct HSL associated with it, so we required two quorum sensing systems which were orthogonal. We found that Las and Rhl had very little crosstalk, indicating that they were good candidates for our system. | |
| − | + | ||
| − | === | + | This part worked as expected, and when we placed GFP downstream of the RhlR promoter, we saw fluorescence when we induced with C4 AHL. |
| + | ===Characterisation of BBa_K1893003=== | ||
| + | [[File:IC16_BBa_K1893003_characterisation.png|700px|center|]] | ||
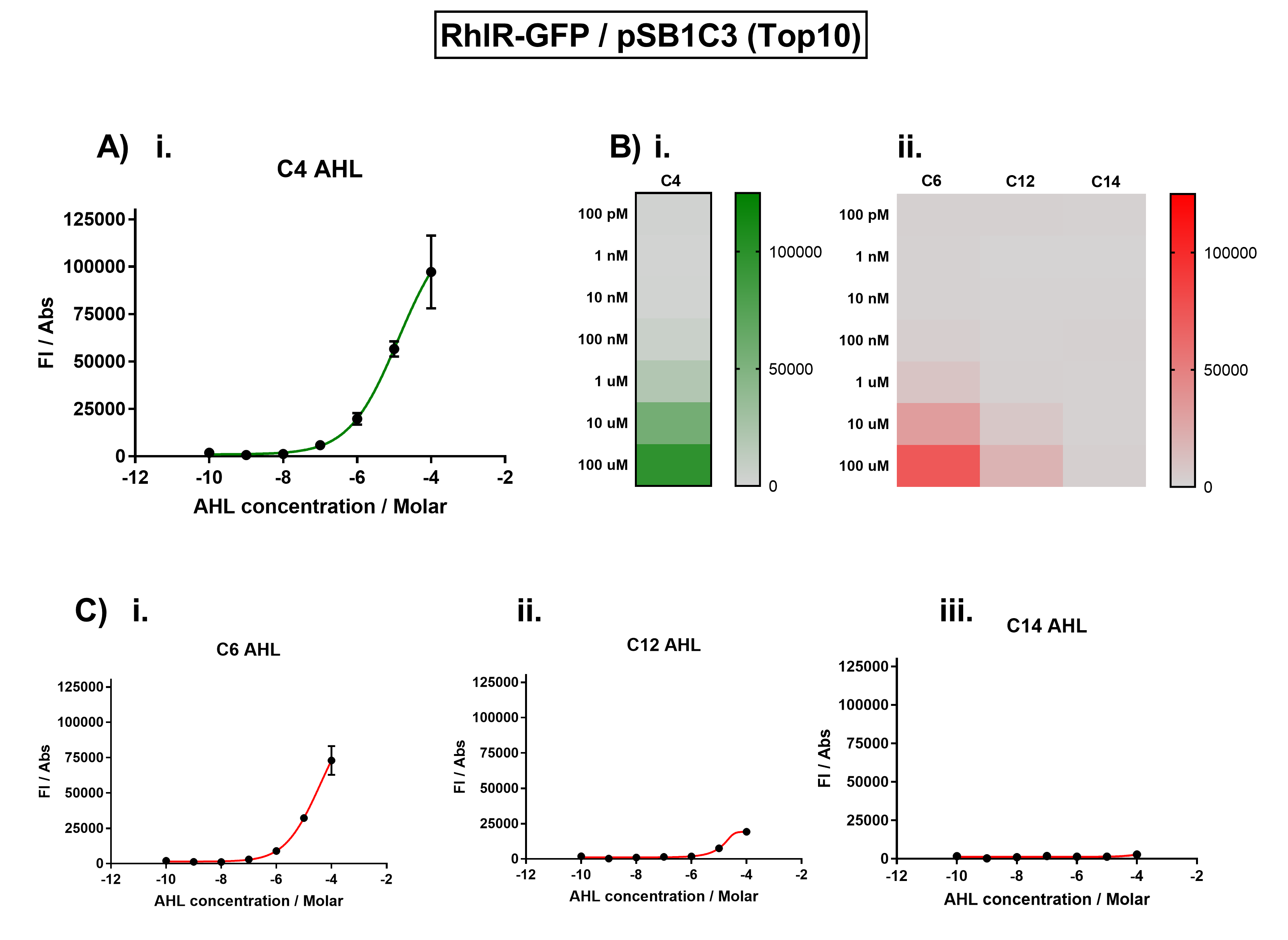
| + | Figure 1. Characterisation of the Rhl response device [https://parts.igem.org/Part:BBa_K1893003 (BBa_K1893003)]. (A) Transfer function curve of normalised fluorescence against cognate inducer C4-AHL concentrations. (B) Heat map of normalised fluorescence of RhlR-GFP system over a range of AHL concentrations: (i) Binding of RhlR-GFP to its cognate AHL (C4 AHL). (ii) Binding of RhlR-GFP to 3 non-cognate AHLs (3O-C6 AHL, 3O-C12 AHL, 3OH-C14 AHL). (C) Transfer function curves of normalised fluorescence against non-cognate inducer AHL (C4 AHL) concentrations to investigate inducer AHL crosstalk: (i) C6-AHL (3O-C6 AHL) of the Lux system (ii) C12-AHL (3O-C12 AHL) of the Las system (iii) C14-AHL (3O-C14 AHL) of the Cin system. Experiments were performed in E. coli Top10 cell strain cultured at 37°C. Normalised fluorescence was calculated by dividing fluorescent signal by cell density (OD600). Fluorescence measurements were recorded at 180 minutes. Reported values represent the mean normalised fluorescence value from 3 technical repeats and error bars represent standard deviation of these. | ||
===User Reviews=== | ===User Reviews=== | ||
Latest revision as of 15:20, 27 October 2016
We used this part to engineer a bacterial communication system which was bidirectional, so that two different strains could grow together. Each strain in our system must have a distinct HSL associated with it, so we required two quorum sensing systems which were orthogonal. We found that Las and Rhl had very little crosstalk, indicating that they were good candidates for our system.
This part worked as expected, and when we placed GFP downstream of the RhlR promoter, we saw fluorescence when we induced with C4 AHL.
Characterisation of BBa_K1893003
Figure 1. Characterisation of the Rhl response device (BBa_K1893003). (A) Transfer function curve of normalised fluorescence against cognate inducer C4-AHL concentrations. (B) Heat map of normalised fluorescence of RhlR-GFP system over a range of AHL concentrations: (i) Binding of RhlR-GFP to its cognate AHL (C4 AHL). (ii) Binding of RhlR-GFP to 3 non-cognate AHLs (3O-C6 AHL, 3O-C12 AHL, 3OH-C14 AHL). (C) Transfer function curves of normalised fluorescence against non-cognate inducer AHL (C4 AHL) concentrations to investigate inducer AHL crosstalk: (i) C6-AHL (3O-C6 AHL) of the Lux system (ii) C12-AHL (3O-C12 AHL) of the Las system (iii) C14-AHL (3O-C14 AHL) of the Cin system. Experiments were performed in E. coli Top10 cell strain cultured at 37°C. Normalised fluorescence was calculated by dividing fluorescent signal by cell density (OD600). Fluorescence measurements were recorded at 180 minutes. Reported values represent the mean normalised fluorescence value from 3 technical repeats and error bars represent standard deviation of these.
User Reviews
UNIQ9dcd194a103d67a3-partinfo-00000000-QINU UNIQ9dcd194a103d67a3-partinfo-00000001-QINU