Difference between revisions of "Part:BBa K1592000"
| (5 intermediate revisions by the same user not shown) | |||
| Line 27: | Line 27: | ||
<h1>Improvement</h1> | <h1>Improvement</h1> | ||
<p> | <p> | ||
| − | HUST-China 2015 put up | + | HUST-China 2015 put up an original method to cement sands as a promising way to help build firm structure in marine environment. The project “Euk.cement” was nominated “Best Environment Project” and “Best New Basic Part”. After the Jamboree, HUST-China iGEMers stepped forward--We did more part characterizations and achieved more valid data to submit to the registry. What’s more exciting is that we successfully published a paper “A living eukaryotic auto-cementation kit from surface display of silica binding peptides on Yarrowia lipolytica” on ACS Synthetic Biology (IF 6.076). We will demonstrate our working details in the following: |
</p> | </p> | ||
<h2>Flocculation system</h2> | <h2>Flocculation system</h2> | ||
<p> | <p> | ||
Last year we ran a SDS-PAGE to identify the flocculating protein MCFP3. Moreover, we tested its function by applying the concentrated supernatant on an object slide and Microscopic observation after staining. The microscopy result qualitatively indicated the successfully secretion of MCFP3. | Last year we ran a SDS-PAGE to identify the flocculating protein MCFP3. Moreover, we tested its function by applying the concentrated supernatant on an object slide and Microscopic observation after staining. The microscopy result qualitatively indicated the successfully secretion of MCFP3. | ||
| − | This year, we made a further step-- BCA(bicinchoninic acid) quantification methods to describe the MCFP3 secretion level. We took wild Yarrowia lipolytica | + | This year, we made a further step-- BCA (bicinchoninic acid) quantification methods to describe the MCFP3 secretion level. We took wild Yarrowia lipolytica culture as control group to verify the proportion that MCFP3 accounted for in all secreted proteins. It was noteworthy that BCA quantification of the total protein in concentrated cell culture suspensions showed that the engineered cells released a protein level 0.1 µg/µL higher than that of the control group. |
</p> | </p> | ||
| − | [[File:T-HUST-China--Improvement-Fig1.jpg| | + | <br> |
| + | [[File:T-HUST-China--Improvement-Fig1.jpg|800px|thumb|center|Fig1: BCA (bicinchoninic acid) quantification of MCFP3's secretion level.]] | ||
<h2>Sand cementation function</h2> | <h2>Sand cementation function</h2> | ||
<p> | <p> | ||
| − | Last year, because of time | + | Last year, because of the limited time, we only tested sand cementation function of the ST123-JMY1212&mcfp3-JMY1212 mixed cells. It showed obvious effect on cementing sands. |
| − | To make the data valid, this year, we together characterized 8 combinations of Si-tag domains and MCFP3 producing cells, and the | + | To make the data valid, this year, we together characterized 8 combinations of Si-tag domains and MCFP3 producing cells, and the results were quite corresponding to our expectation. |
| − | The cementation test verified the cooperation of immobilization system and flocculation system cells in actual application conditions. Quartz sand (40 grams) mixed with either Si-tag+MCFP3 YMY1212 cells or control wild-type cells was loaded into a glass column, and a solution carrying oxygen, calcium and culture nutrients was supplied into the column using a peristaltic pump (Figure 4a). After 24 hours of treatment, the sand columns were dehydrated in a drying oven and then removed from the glass column. The sand treated with control wild-type JMY1212 cells was still scattered; only a few small clumps could be identified (Figure 4 c), and these may have been induced by the constitutive respiration of the wild type cells. However, with the treatment of Si-tag+MCFP3 cells, the sand aggregated, and an intact solid sand cylinder was obtained (Figure 4b). With further comparison of the treated sand under a microscope, the quartz sand granules treated with Si-tag+MCFP3 cells were found to be tightly agglutinated, whereas the quartz sand granules treated with wild type cells remained dispersed (Figure 4 d, e). | + | The cementation test verified the cooperation of immobilization system and flocculation system cells in actual application conditions. Quartz sand (40 grams) mixed with either Si-tag+MCFP3 YMY1212 cells or control wild-type cells was loaded into a glass column, and a solution carrying oxygen, calcium and culture nutrients was supplied into the column using a peristaltic pump (Figure 4a). After 24 hours of treatment, the sand columns were dehydrated in a drying oven and then removed from the glass column. The sand treated with control wild-type JMY1212 cells was still scattered; only a few small clumps could be identified (Figure 4 c), and these may have been induced by the constitutive respiration of the wild type cells. However, with the treatment of Si-tag+MCFP3 cells, the sand aggregated, and an intact solid sand cylinder was obtained (Figure 4b). With further comparison of the treated sand under a microscope, the quartz sand granules treated with Si-tag+MCFP3 cells were found to be tightly agglutinated, whereas the quartz sand granules treated with wild type cells remained dispersed (Figure 4 d, e).</p> |
| − | + | <p>These results indicated that Si-tag+MCFP3 cells actually worked well at making silica particles form a certain intact structure, which fits our hypothesis and design of their cementation function. It was also noticed in the cementation test that there were some small holes in the cemented sand cylinder. This special porous structure indicated the balance between the CO2 released from cell respiration and the calcium/magnesium sedimentation caused by the released CO2. This sedimentation, however, will be the final and vital step of the cementation process. Indeed, in some cementation applications, this structure is very important. For example, in desert sand consolidation treatment, this multi-porous structure will eliminate the potential compaction risk and will enable organisms to grow on it; in artificial reef construction on aqua farms, the multi-porous structure could also offer niches to all types of marine life. | |
</p> | </p> | ||
| − | [[File:T-HUST-China--Improvement-Fig4.jpg| | + | <br> |
| − | + | [[File:T-HUST-China--Improvement-Fig4.jpg|800px|thumb|center|Figure 4: Sand cementation test with Si-tag and MCFP3 producing cells. (a) Test facility for sand cementation in lab with trial column and quartz sand. (b) Sand treated with Si-tag and MCFP3 producing cells formed cementation in the column, (c) whereas sand treated with wild type control JMY1212 cells did not form cementation. (d) Sand particles from the Si-tag and MCFP3 producing cell treated column were evaluated using microscopy and were found to be stuck together, (e) but sand particles from the wild type control JMY1212 cell treated column did not stick together. (f) Microscopy image of sand treated with Euk.cement cells in flasks on a shaker, which mimics the real conditions of high water-to-sand ratio and turbulence-like waves. (g) Sand treated with control cells in flasks on a shaker. (h) Sand treated with Euk.cement cells in column forms a cemented cylinder. (i) Standardized 1cm3 cube was modified from cementation sand cylinder and put on a platform weight scale. Weight was added onto the cube and the critical pressure value at cube destruction was recorded, and then normalized by the highest value. Quantification showing the different intensity of cylinders from the cementation of sand treated with different cells (quantification: n=3, t-test *: P<0.05)]] | |
<p> | <p> | ||
| − | To mimic the real conditions in underwater applications, we also tested the sand cementation under the condition of high water-to-sand ratio with turbulence in flasks on a shaker. Compared to sand treated with wild-type cells, sand treated with Si-tag cells and MCFP3 cells was found to be cemented together tightly using microscopy (Figure 4f, g). | + | To mimic the real conditions in underwater applications, we also tested the sand cementation under the condition of high water-to-sand ratio with turbulence in flasks on a shaker. Compared to sand treated with wild-type cells, sand treated with Si-tag cells and MCFP3 cells was found to be cemented together tightly using microscopy (Figure 4f,g). |
To find whether sand treated with different Si-tag cells can form cementation with different intensity, column cementation tests were also conducted with MCFP3 cells and different Si-tag cells. Sand treated with all Si-tag cells except wild-type control cells could form a cemented cylinder (Figure 4h). The relative intensity of the cylinders was quantified by the critical pressure value at cementation destruction. The results showed that Si-tag1+2+3 provided the highest cementation intensity, whereas Si-tag2+3 provided medium cementation intensity and the other strains provided weak cementation intensity (Figure 4i). This finding is comparable to the results from the Si-tag silica binding test in which Si-tag1+2+3 cells provided the highest silica binding intensity while other cells provided medium or weak intensity. | To find whether sand treated with different Si-tag cells can form cementation with different intensity, column cementation tests were also conducted with MCFP3 cells and different Si-tag cells. Sand treated with all Si-tag cells except wild-type control cells could form a cemented cylinder (Figure 4h). The relative intensity of the cylinders was quantified by the critical pressure value at cementation destruction. The results showed that Si-tag1+2+3 provided the highest cementation intensity, whereas Si-tag2+3 provided medium cementation intensity and the other strains provided weak cementation intensity (Figure 4i). This finding is comparable to the results from the Si-tag silica binding test in which Si-tag1+2+3 cells provided the highest silica binding intensity while other cells provided medium or weak intensity. | ||
</p> | </p> | ||
Latest revision as of 07:00, 25 October 2016
LIP2 prepro(signal peptide)
13 aa pre/4 XA or XP dipeptides/10 aa pro/KR cleavage site
The pre-region of lipase 2 from Yarrowia lipolytica corresponds to the signal sequence.
LIP2 prepro consist of 13aa pre-region of lipase2, four XA or XP dipeptides, 10aa pro-region of lipase2, and KR cleavage site.
the dipeptides XA and XP are substrates for diamino-peptidase; the dibasic KR cleavage site is substrate for Xpr6p endoproteinase.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Application of the part
This signal tag, LIP2 prepro, fused to Mcfp3 and constructed a new biobrick (BBa_K1592003), can lead the co-translational translocation of heterologous protein Mcfp-3 to be secreted out of the cell to achieve its function.
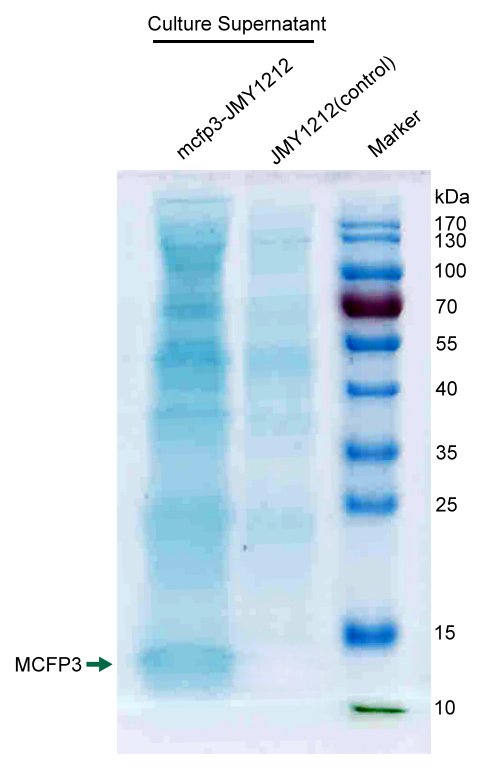
We concentrated cell culture solution of test Y.lipolytca JMY1212 cells transformed LIP2 prepro-Mcfp3 plasmid and control wildtype cells, and then separated proteins by SDS-PAGE.
Figure shows an obvious ~12kDa protein bands of LIP2 prepro-Mcfp3 in test lane, which cannot be found in control lane. This result proves that LIP2 prepro signal peptide can successfully lead the flocculating proteins Mcfp3 into surrounding environment.
Improvement
HUST-China 2015 put up an original method to cement sands as a promising way to help build firm structure in marine environment. The project “Euk.cement” was nominated “Best Environment Project” and “Best New Basic Part”. After the Jamboree, HUST-China iGEMers stepped forward--We did more part characterizations and achieved more valid data to submit to the registry. What’s more exciting is that we successfully published a paper “A living eukaryotic auto-cementation kit from surface display of silica binding peptides on Yarrowia lipolytica” on ACS Synthetic Biology (IF 6.076). We will demonstrate our working details in the following:
Flocculation system
Last year we ran a SDS-PAGE to identify the flocculating protein MCFP3. Moreover, we tested its function by applying the concentrated supernatant on an object slide and Microscopic observation after staining. The microscopy result qualitatively indicated the successfully secretion of MCFP3. This year, we made a further step-- BCA (bicinchoninic acid) quantification methods to describe the MCFP3 secretion level. We took wild Yarrowia lipolytica culture as control group to verify the proportion that MCFP3 accounted for in all secreted proteins. It was noteworthy that BCA quantification of the total protein in concentrated cell culture suspensions showed that the engineered cells released a protein level 0.1 µg/µL higher than that of the control group.
Sand cementation function
Last year, because of the limited time, we only tested sand cementation function of the ST123-JMY1212&mcfp3-JMY1212 mixed cells. It showed obvious effect on cementing sands. To make the data valid, this year, we together characterized 8 combinations of Si-tag domains and MCFP3 producing cells, and the results were quite corresponding to our expectation. The cementation test verified the cooperation of immobilization system and flocculation system cells in actual application conditions. Quartz sand (40 grams) mixed with either Si-tag+MCFP3 YMY1212 cells or control wild-type cells was loaded into a glass column, and a solution carrying oxygen, calcium and culture nutrients was supplied into the column using a peristaltic pump (Figure 4a). After 24 hours of treatment, the sand columns were dehydrated in a drying oven and then removed from the glass column. The sand treated with control wild-type JMY1212 cells was still scattered; only a few small clumps could be identified (Figure 4 c), and these may have been induced by the constitutive respiration of the wild type cells. However, with the treatment of Si-tag+MCFP3 cells, the sand aggregated, and an intact solid sand cylinder was obtained (Figure 4b). With further comparison of the treated sand under a microscope, the quartz sand granules treated with Si-tag+MCFP3 cells were found to be tightly agglutinated, whereas the quartz sand granules treated with wild type cells remained dispersed (Figure 4 d, e).
These results indicated that Si-tag+MCFP3 cells actually worked well at making silica particles form a certain intact structure, which fits our hypothesis and design of their cementation function. It was also noticed in the cementation test that there were some small holes in the cemented sand cylinder. This special porous structure indicated the balance between the CO2 released from cell respiration and the calcium/magnesium sedimentation caused by the released CO2. This sedimentation, however, will be the final and vital step of the cementation process. Indeed, in some cementation applications, this structure is very important. For example, in desert sand consolidation treatment, this multi-porous structure will eliminate the potential compaction risk and will enable organisms to grow on it; in artificial reef construction on aqua farms, the multi-porous structure could also offer niches to all types of marine life.

To mimic the real conditions in underwater applications, we also tested the sand cementation under the condition of high water-to-sand ratio with turbulence in flasks on a shaker. Compared to sand treated with wild-type cells, sand treated with Si-tag cells and MCFP3 cells was found to be cemented together tightly using microscopy (Figure 4f,g). To find whether sand treated with different Si-tag cells can form cementation with different intensity, column cementation tests were also conducted with MCFP3 cells and different Si-tag cells. Sand treated with all Si-tag cells except wild-type control cells could form a cemented cylinder (Figure 4h). The relative intensity of the cylinders was quantified by the critical pressure value at cementation destruction. The results showed that Si-tag1+2+3 provided the highest cementation intensity, whereas Si-tag2+3 provided medium cementation intensity and the other strains provided weak cementation intensity (Figure 4i). This finding is comparable to the results from the Si-tag silica binding test in which Si-tag1+2+3 cells provided the highest silica binding intensity while other cells provided medium or weak intensity.