Difference between revisions of "Part:BBa K1926013"
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
<partinfo>BBa_K1926013 short</partinfo> | <partinfo>BBa_K1926013 short</partinfo> | ||
| − | This part is the mCHERRY UNIT of cyclebow system including a degradation tag named DBOX, a fluorescent gene mCherry and an sv40 terminator flanked by homodromous recognition site of recombinase VIKA named Vox. The whole part can be cut off by VIKA. This part is the improvement of the [[Part: BBa_J06504]],which is a plain mCherry. | + | This part is the mCHERRY UNIT of cyclebow system including a degradation tag named DBOX, a fluorescent gene mCherry and an sv40 terminator flanked by homodromous recognition site of recombinase VIKA named Vox. The whole part can be cut off by VIKA. This part is the improvement of the [[Part:BBa_J06504]],which is a plain mCherry. |
| − | + | ||
| Line 28: | Line 27: | ||
The mCherry with tags and RTSs are constructed using PCR and oligonucleotide ligation by the following procedure. Our UNITs all contain XbaI and SpeI enzyme sites, so we can easily line up the UNITs using 3A assembly. | The mCherry with tags and RTSs are constructed using PCR and oligonucleotide ligation by the following procedure. Our UNITs all contain XbaI and SpeI enzyme sites, so we can easily line up the UNITs using 3A assembly. | ||
| − | [[File:5-连接过程改.png| | + | [[File:5-连接过程改.png|600px|thumb|left|'''Figure 1:''' Three steps to construct our UNITs using PCR and oligonucleotide ligation.]] |
<br style="clear: both" /> | <br style="clear: both" /> | ||
| + | |||
| + | ===Gel analysis after EcoR1 digestion=== | ||
| + | |||
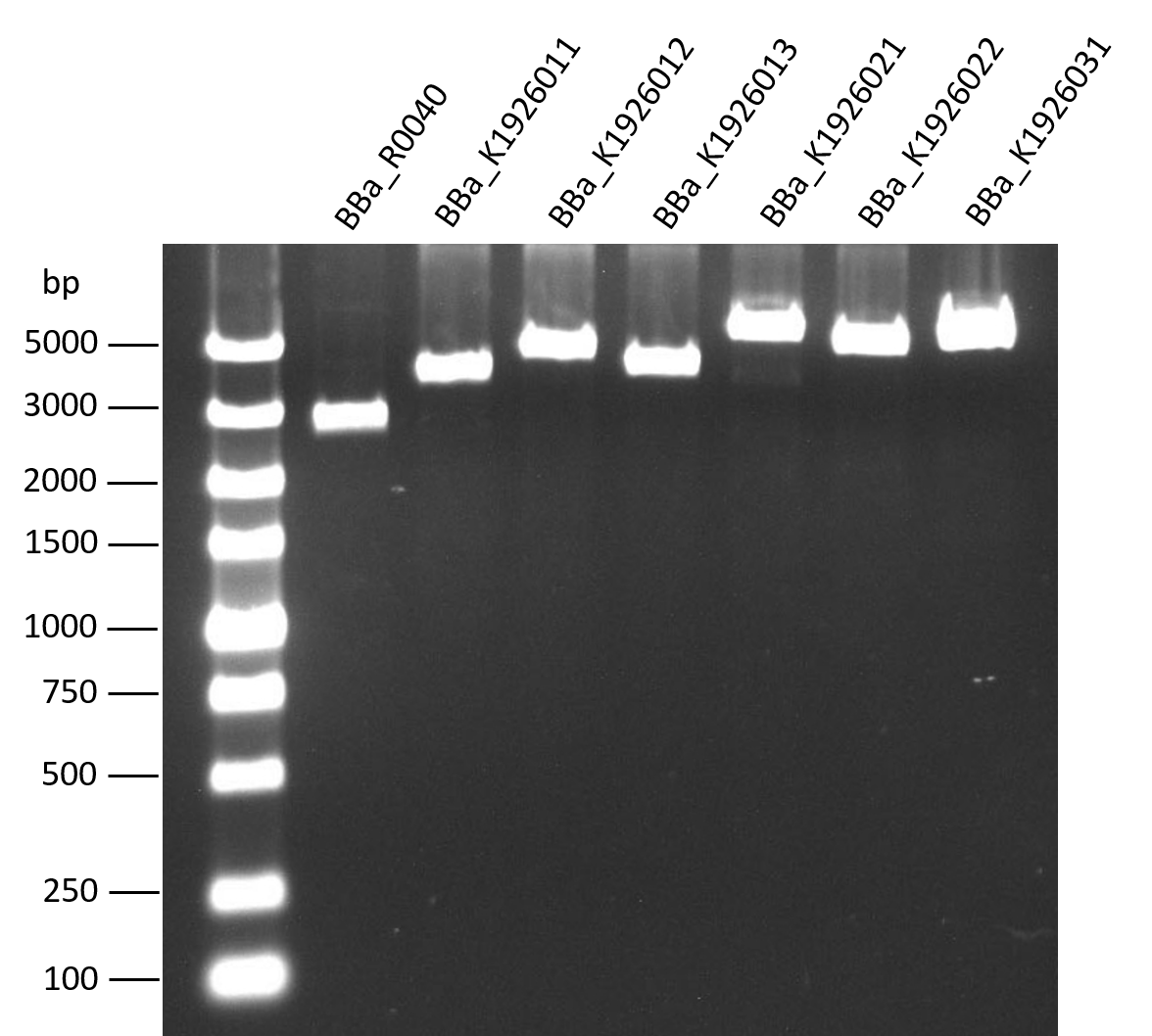
| + | The length of our biobricks are confirmed by gel analysis after EcoR1 digestion. The biobrick BBa_R0040, which is the negative control in 2016’s interlab, was used as a control because it only have a 54 bp part. | ||
| + | |||
| + | [[File: 2016--SYSU-CHINA--biobrick2.png|400px|thumb|left|'''Figure 2:''' AGE image of biobricks BBa_K1926001-BBa_K1926003. The samples were pre dyed with gelred]] | ||
| + | <br style="clear:both" /> | ||
Latest revision as of 07:31, 19 October 2016
The mCHERRY UNIT: mCherry flanked by Vox
This part is the mCHERRY UNIT of cyclebow system including a degradation tag named DBOX, a fluorescent gene mCherry and an sv40 terminator flanked by homodromous recognition site of recombinase VIKA named Vox. The whole part can be cut off by VIKA. This part is the improvement of the Part:BBa_J06504,which is a plain mCherry.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage
You may use this part to:
1) Provide fluorescent red which can be quickly cut off by transient transfecting recombinase gene into nucleus;
2) Use it as a part of the flp-out system[1].
Source
The sequence of Vox and DBOX was retrieved from Addgene and got through denovo synthesis. mCHERRY and sv40 terminator were got from commercial plasmid: pentry dcas9 mcherry bira and pentry nls GFP/pcdna3.1, respectfully.
Construction Design
The mCherry with tags and RTSs are constructed using PCR and oligonucleotide ligation by the following procedure. Our UNITs all contain XbaI and SpeI enzyme sites, so we can easily line up the UNITs using 3A assembly.
Gel analysis after EcoR1 digestion
The length of our biobricks are confirmed by gel analysis after EcoR1 digestion. The biobrick BBa_R0040, which is the negative control in 2016’s interlab, was used as a control because it only have a 54 bp part.
Reference
1. Voutev, R. and E.J. Hubbard, A "FLP-Out" system for controlled gene expression in Caenorhabditis elegans. Genetics, 2008. 180(1): p. 103-19.