Difference between revisions of "Part:BBa K1680009"
KatharinaS (Talk | contribs) |
Kateesc1700 (Talk | contribs) |
||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K1680009 short</partinfo> | <partinfo>BBa_K1680009 short</partinfo> | ||
| − | This part contains the protein coding region for the NanoLuc(tm) luciferase, which is originally designed by Promega Corporation (http://bit.ly/1Vgmab3, free for research use). We codon-optimized the part for yeast. The NanoLuc is smaller than other luciferases while yielding equal or stronger luminescence readings. | + | This part contains the protein coding region for the NanoLuc(tm) luciferase, which is originally designed by Promega Corporation (http://bit.ly/1Vgmab3, free for research use). |
| + | Nanoluciferase is a small and monomeric enzyme (19.1kDa) and is about 150 times brighter than other luciferases. The new substrate called furimazine produces a high intensity luminescence. | ||
| + | |||
| + | |||
| + | |||
| + | |||
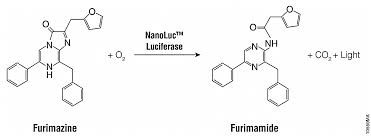
| + | [[File:nanoluciferase.jpeg|300px|thumb|left|Figure 1 : Nanoluciferase reaction with its substrate called furimazine. The enzyme creates an oxidative process. This picture was taken on the Promega’s site. | ||
| + | source : PDF https://promega.com>promega uk>teddy-riss-02 | ||
| + | ]] | ||
| + | |||
| + | |||
| + | |||
| + | After looking at the bibliography, Nanoluciferase gene was chosen because it allowed us to have a stronger signal. Indeed, this protein isn’t secreted but unfused. Furthermore, its intracellular half-life is superior to 6 hours and the Nanoluciferase has a low autoluminescence. This gives us maximum sensitivity. | ||
| + | NLuc is used in biomedical research for several applications, including studying protein-protein interactions, monitoring protein stability, BRET-based sensors, molecular imaging or investigating genetic regulation and cell signaling. | ||
| + | The emission pic of nanoluciferase is located at 460nm. | ||
| + | We codon-optimized the part for yeast. The NanoLuc is smaller than other luciferases while yielding equal or stronger luminescence readings. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| Line 11: | Line 34: | ||
[[Image:Team Tuebingen mpr 6h.jpg|400px]] | [[Image:Team Tuebingen mpr 6h.jpg|400px]] | ||
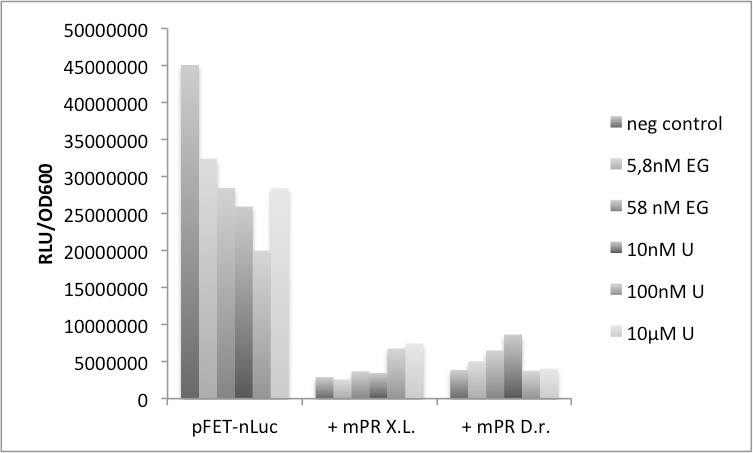
| − | RLU measurement of the NanoLuc under pFET3 promoter [[BBa_K950000]] control, normalized to OD600. | + | RLU measurement of the NanoLuc under pFET3 promoter [[part:BBa_K950000]] control, normalized to OD600. |
| + | <h2> MilkClear iGEM UCopenhagen 2024 </h2> | ||
| + | |||
| + | Author: Kate Escobar, 26-09-2024, MilkClear's Wiki: https://2024.igem.wiki/ucopenhagen/ | ||
| + | |||
| + | This part was used in the reporter modules [https://parts.igem.org/Part:BBa_K5477030| BBa_K5477030] and [https://parts.igem.org/Part:BBa_K5477031| BBa_K5477031] to build the following devices [https://parts.igem.org/Part:BBa_K5477041| BBa_K5477041], [https://parts.igem.org/Part:BBa_K5477042| BBa_K5477042], [https://parts.igem.org/Part:BBa_K5477043| BBa_K5477043], [https://parts.igem.org/Part:BBa_K5477044| BBa_K5477044], [https://parts.igem.org/Part:BBa_K5477045| BBa_K5477045] and [https://parts.igem.org/Part:BBa_K5477046| BBa_K5477046]. | ||
| + | |||
| + | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 20: | Line 50: | ||
<!-- --> | <!-- --> | ||
| − | <span class='h3bb'>Sequence and Features</span> | + | <span class='h3bb'>'''Sequence and Features'''</span> |
<partinfo>BBa_K1680009 SequenceAndFeatures</partinfo> | <partinfo>BBa_K1680009 SequenceAndFeatures</partinfo> | ||
Latest revision as of 21:16, 1 October 2024
NanoLuc Luciferase
This part contains the protein coding region for the NanoLuc(tm) luciferase, which is originally designed by Promega Corporation (http://bit.ly/1Vgmab3, free for research use). Nanoluciferase is a small and monomeric enzyme (19.1kDa) and is about 150 times brighter than other luciferases. The new substrate called furimazine produces a high intensity luminescence.
After looking at the bibliography, Nanoluciferase gene was chosen because it allowed us to have a stronger signal. Indeed, this protein isn’t secreted but unfused. Furthermore, its intracellular half-life is superior to 6 hours and the Nanoluciferase has a low autoluminescence. This gives us maximum sensitivity. NLuc is used in biomedical research for several applications, including studying protein-protein interactions, monitoring protein stability, BRET-based sensors, molecular imaging or investigating genetic regulation and cell signaling. The emission pic of nanoluciferase is located at 460nm. We codon-optimized the part for yeast. The NanoLuc is smaller than other luciferases while yielding equal or stronger luminescence readings.
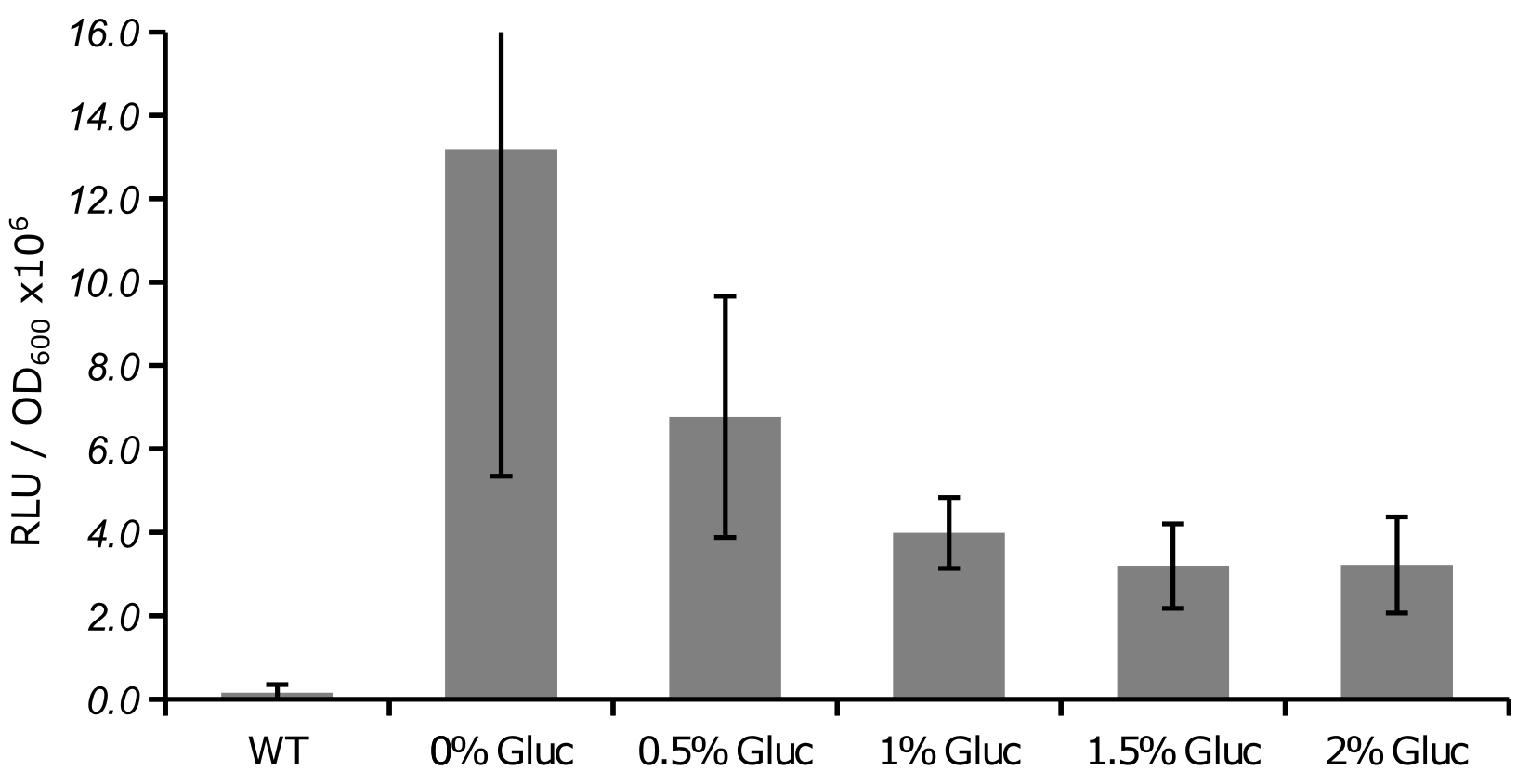
RLU measurement of the NanoLuc under pSUC2 promoter part:BBa_K950003 control, normalized to OD600.
RLU measurement of the NanoLuc under pFET3 promoter part:BBa_K950000 control, normalized to OD600.
MilkClear iGEM UCopenhagen 2024
Author: Kate Escobar, 26-09-2024, MilkClear's Wiki: https://2024.igem.wiki/ucopenhagen/
This part was used in the reporter modules BBa_K5477030 and BBa_K5477031 to build the following devices BBa_K5477041, BBa_K5477042, BBa_K5477043, BBa_K5477044, BBa_K5477045 and BBa_K5477046.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]