Difference between revisions of "Part:BBa K1758344"
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
<partinfo>BBa_K1758344 short</partinfo> | <partinfo>BBa_K1758344 short</partinfo> | ||
| − | + | Mercury inducible promoter under the control of a T7 promoter with 5´untranslated region and sfGFP for detection | |
===Usage and Biology=== | ===Usage and Biology=== | ||
<html> | <html> | ||
| − | <p>For the in vitro characterization we used a cell extract out of cells which contain the | + | <p>The promoter <i>PmerT</i> is regulated by the MerR, which binds Hg2+-ions. Similar to the former sensors we added sfGFP for detection via fluorescence. It is based on BBa_K346001 designed by team Peking 2010. For the in vitro characterization we used a cell extract out of cells which contain the plasmid (<a href="https://parts.igem.org/Part:BBa_K1758340" target="_blank"> BBa_K1758340</a>). In addition to that we added plasmid DNA of the mercury specific promoter merT with 5’UTR-sfGFP under the control of T7-promoter (<a href="https://parts.igem.org/Part:BBa_K1758344" target="_blank"> BBa_K1758344</a>)to the cell extract. The T7 promoter is needed to get a better expression. </p> |
</html> | </html> | ||
| Line 19: | Line 19: | ||
<html> | <html> | ||
<h2><i>in vitro</i></h2> | <h2><i>in vitro</i></h2> | ||
| − | <p>For the characterization of the mercury sensor with CFPS we used parts differing from that we used in the <i>in vivo</i> characterization. For the <i>in vitro</i> characterization we used a cell extract out of cells, which contained the plasmid (<a href="https://parts.igem.org/Part:BBa_K1758340" target="_blank"> BBa_K1758340</a>)(figure 5). In addition, we added plasmid DNA to the cell extract. This plasmid consisted of the mercury specific promoter <i>pmerT</i> with 5’-UTR-sfGFP. The entire sequence was placed under the control of of T7 | + | <p>For the characterization of the mercury sensor with CFPS we used parts differing from that we used in the <i>in vivo</i> characterization. For the <i>in vitro</i> characterization we used a cell extract out of cells, which contained the plasmid (<a href="https://parts.igem.org/Part:BBa_K1758340" target="_blank"> BBa_K1758340</a>)(figure 5). In addition, we added plasmid DNA to the cell extract. This plasmid consisted of the mercury specific promoter <i>pmerT</i> with 5’-UTR-sfGFP. The entire sequence was placed under the control of of T7 promoter (<a href="https://parts.igem.org/Part:BBa_K1758344" target="_blank"> BBa_K1758344</a>)(figure 6). The T7-promoter is needed to get a better fluorescence expression.</p> |
<div class="row"> | <div class="row"> | ||
| Line 56: | Line 56: | ||
<p><i>In vitro</i> this sensor showed good results. The fluorescence level was high at low concentrations. Additionally, it showed that the expression level at 6 µg/L (Guideline of WHO for Mercury) reached the maximal signal. This result indicated the potential for measurement of concentrations under 6 µg/L.To confirm this hypothesis, it takes more experiments and tests with lower concentrations. Due to the high expression of sfGFP at low concentrations and the same expression level at different concentrations, it is not possible to quantify mercury with CFPS analyses . , Our model predicted this observation. During the measurement we noticed that the heavy metals have negative influences on the cell extract. Because of this fact, we used a correction factor, which resulted from the heavy metals influence on the CFPS system. This already optimized sensor showed the high potential of optimized sensors in CFPS.</p> | <p><i>In vitro</i> this sensor showed good results. The fluorescence level was high at low concentrations. Additionally, it showed that the expression level at 6 µg/L (Guideline of WHO for Mercury) reached the maximal signal. This result indicated the potential for measurement of concentrations under 6 µg/L.To confirm this hypothesis, it takes more experiments and tests with lower concentrations. Due to the high expression of sfGFP at low concentrations and the same expression level at different concentrations, it is not possible to quantify mercury with CFPS analyses . , Our model predicted this observation. During the measurement we noticed that the heavy metals have negative influences on the cell extract. Because of this fact, we used a correction factor, which resulted from the heavy metals influence on the CFPS system. This already optimized sensor showed the high potential of optimized sensors in CFPS.</p> | ||
| − | + | <b>References</b> | |
| + | <p>iGEM Team Peking 2010</p> | ||
| + | <p> Brown, Nigel L.; Stoyanov, , Jivko V.;Kidd,Stephen P.;Hobman; Jon L. (2003): The MerR family of transcriptional regulators. In FEMS Microbiology Reviews, 27 ( 2) pp.145-163.</p> | ||
</html> | </html> | ||
Latest revision as of 13:33, 7 March 2019
Mercury responsive promoter with T7-promoter and UTR-sfGFP
Mercury inducible promoter under the control of a T7 promoter with 5´untranslated region and sfGFP for detection
Usage and Biology
The promoter PmerT is regulated by the MerR, which binds Hg2+-ions. Similar to the former sensors we added sfGFP for detection via fluorescence. It is based on BBa_K346001 designed by team Peking 2010. For the in vitro characterization we used a cell extract out of cells which contain the plasmid ( BBa_K1758340). In addition to that we added plasmid DNA of the mercury specific promoter merT with 5’UTR-sfGFP under the control of T7-promoter ( BBa_K1758344)to the cell extract. The T7 promoter is needed to get a better expression.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 145
in vitro
For the characterization of the mercury sensor with CFPS we used parts differing from that we used in the in vivo characterization. For the in vitro characterization we used a cell extract out of cells, which contained the plasmid ( BBa_K1758340)(figure 5). In addition, we added plasmid DNA to the cell extract. This plasmid consisted of the mercury specific promoter pmerT with 5’-UTR-sfGFP. The entire sequence was placed under the control of of T7 promoter ( BBa_K1758344)(figure 6). The T7-promoter is needed to get a better fluorescence expression.




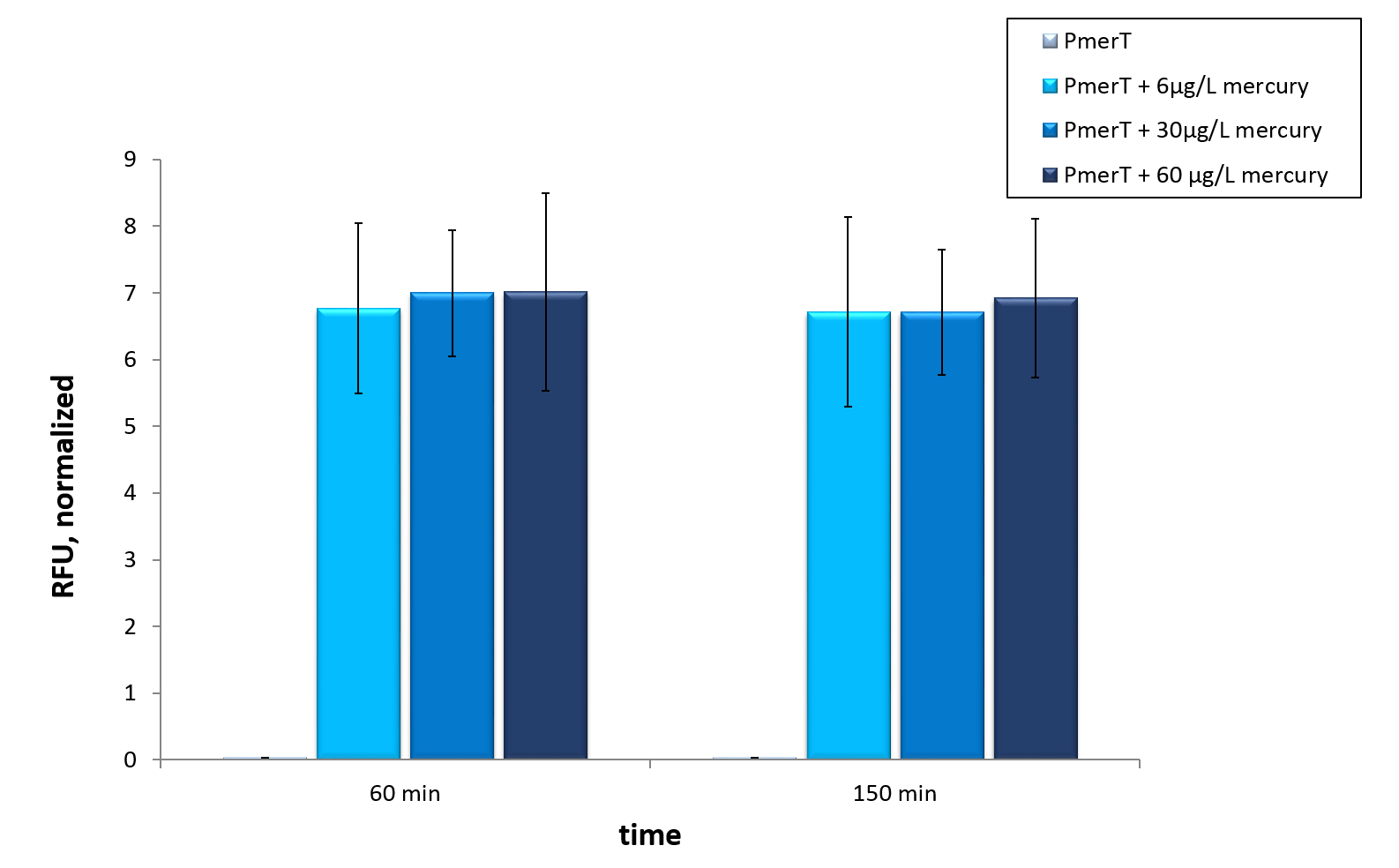
In vitro this sensor showed good results. The fluorescence level was high at low concentrations. Additionally, it showed that the expression level at 6 µg/L (Guideline of WHO for Mercury) reached the maximal signal. This result indicated the potential for measurement of concentrations under 6 µg/L.To confirm this hypothesis, it takes more experiments and tests with lower concentrations. Due to the high expression of sfGFP at low concentrations and the same expression level at different concentrations, it is not possible to quantify mercury with CFPS analyses . , Our model predicted this observation. During the measurement we noticed that the heavy metals have negative influences on the cell extract. Because of this fact, we used a correction factor, which resulted from the heavy metals influence on the CFPS system. This already optimized sensor showed the high potential of optimized sensors in CFPS.
ReferencesiGEM Team Peking 2010
Brown, Nigel L.; Stoyanov, , Jivko V.;Kidd,Stephen P.;Hobman; Jon L. (2003): The MerR family of transcriptional regulators. In FEMS Microbiology Reviews, 27 ( 2) pp.145-163.
