Difference between revisions of "Part:BBa K1758342"
| (One intermediate revision by one other user not shown) | |||
| Line 2: | Line 2: | ||
<html> | <html> | ||
Mercury responsive promoter with UTR-sfGFP | Mercury responsive promoter with UTR-sfGFP | ||
| − | This part is essential for our <i>in | + | This part is essential for our <i>in vivo</i> characterization. In combination with <a href="https://parts.igem.org/Part:BBa_K1758342" target="_blank"> BBa_K1758340</a>,<a href="https://parts.igem.org/Part:BBa_K1758340" target="_blank"> BBa_K1758343</a>,<a href="https://parts.igem.org/Part:BBa_K1758344" target="_blank"> BBa_K1758344</a> this device builds our mercury sensor. |
</html> | </html> | ||
| Line 14: | Line 14: | ||
<partinfo>BBa_K1758342 parameters</partinfo> | <partinfo>BBa_K1758342 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | <html> | ||
<h2><i>in vivo</i></h2> | <h2><i>in vivo</i></h2> | ||
Latest revision as of 13:35, 7 March 2019
Usage and Biology
Mercury responsive promoter with UTR-sfGFP This part is essential for our in vivo characterization. In combination with BBa_K1758340, BBa_K1758343, BBa_K1758344 this device builds our mercury sensor.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 122
in vivo
One of the already existing sensors we used for our system is the mercury sensor consisting of MerR the activator and the mercury specific promoter pmerT. The promoter is regulated by the MerR, which binds Hg2+-ions. Similar to the former sensors we added a sfGFP for detection via fluorescence.
For our mercury sensor we used parts of the mercury sensor constructed by iGEM team Peking 2010. These parts consist of the mercury dependent mer operon from Shigella flexneri R100 plasmid Tn21. The expression of the genes in the mer operon depends on the regulation by MerR its activator and promoter PmerT. For our sensor we used the codon optimized activator (BBa_K1758340), under control of a constitutive promoter,(BBa_K346001). Additionally to this activator we designed and constructed the specific promoter PmerT(BBa_K346002)(figure 2). For our sensor we added a 5’-UTR downstreamd of this promoter, which increased the fluorscence of the used reporter protein sfGFP.

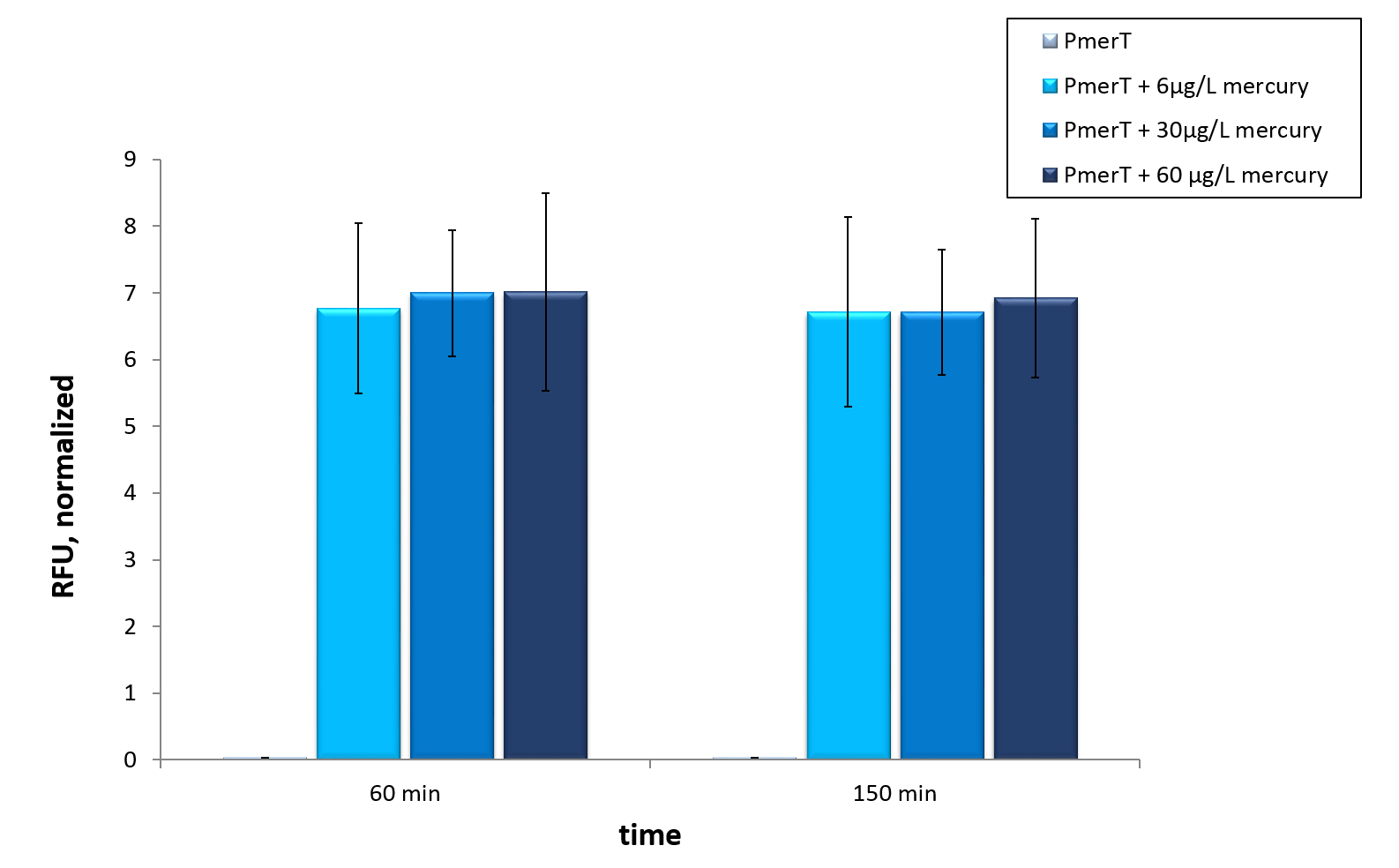
We tested our mercury sensor with sfGFP as reporter gene, to test the functionality of the system. Moreover we tested different concentrations. The kinetic of our sensors response to different mercury concentrations is shown in figure 3. A strong increase in fluorescence levels is notecible after induction with mercury after 120 min. For better visualization the kinetics of figure 3 are represented as bars in figure 4. A fluorescence level difference for 120 min and 190 min is represented.
In vivo data show a highly significant, well working sensor, which even reacts to concentrations below the threshold of the water guidelines by the WHO (Figure 3 and 4).
The mercury detection was measured during the cultivation of E. coli KRX at 37 °C (Figure 3 and 4). The strain contained the plasmid with the activator merR under the control of a constitutive promoter and the specific promoter with an operator binding site, which reacts to the activator with bound Hg 2+-ions. The specific promoter is located upstream of the sfGFP CDS. Therefore, the mercury in the medium is detected via formation of sfGFP. In vivothis sensor devise shows a fast answer to occurrence of his heavy metal contrary to the other sensor systems In vivo.
Therefore we tested our sensor in vitro to check if an already functioning highly optimized sensor provides required data for guideline detections
in vitro
For the characterization of the mercury sensor with CFPS we used parts differing from that we used in the in vivo characterization. For the in vitro characterization we used a cell extract out of cells, which contained the plasmid ( BBa_K1758340)(figure 5). In addition, we added plasmid DNA to the cell extract. This plasmid consisted of the mercury specific promoter pmerT with 5’-UTR-sfGFP. The entire sequence was placed under the control of of T7-promoter ( BBa_K1758344)(figure 6). The T7-promoter is needed to get a better fluorescence expression.




In vitro this sensor showed good results. The fluorescence level was high at low concentrations. Additionally, it showed that the expression level at 6 µg/L (Guideline of WHO for Mercury) reached the maximal signal. This result indicated the potential for measurement of concentrations under 6 µg/L.To confirm this hypothesis, it takes more experiments and tests with lower concentrations. Due to the high expression of sfGFP at low concentrations and the same expression level at different concentrations, it is not possible to quantify mercury with CFPS analyses . , Our model predicted this observation. During the measurement we noticed that the heavy metals have negative influences on the cell extract. Because of this fact, we used a correction factor, which resulted from the heavy metals influence on the CFPS system. This already optimized sensor showed the high potential of optimized sensors in CFPS.
To summarize
We demonstrated, that our mercury sensor works well in vivo (figure 4). There is a clearly noticeable increase in fluorescence after induction with mercury. Even the threshold concentration, which is given in the WHO guideline, can be measured. This well working sensor was tested in in vitro as well. The presented data suggested, that maximal output is reached at concentrations of 6 µg/ L,which represent the value of the former mentioned WHO guideline. Further optimization could lead to a decreasing in vitro detection threshold.


