Difference between revisions of "Part:BBa K1666006"
(→Characterization) |
(→Characterization) |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K1666006 short</partinfo> | <partinfo>BBa_K1666006 short</partinfo> | ||
| − | Quorum sensing is a process of bacterial cell-to-cell communication involving the production and detection of extracellular signaling molecules called autoinducers. And autoinducer-2 (AI-2) has been proposed to serve as a 'universal signal' for interspecies communication. In the LuxS/AI-2 signaling system of Salmonella Typhimurium, AI-2 response involves ATP binding cassette transporter encoded by genes named Lsr (LuxS regulated). P''lsr'' is the promoter of the ''lsr'' operon. In our project, we use it to trigger the expression of a reporter gene. | + | Quorum sensing is a process of bacterial cell-to-cell communication involving the production and detection of extracellular signaling molecules called autoinducers. And autoinducer-2 (AI-2) has been proposed to serve as a 'universal signal' for interspecies communication. In the LuxS/AI-2 signaling system of ''Salmonella Typhimurium'', AI-2 response involves ATP binding cassette transporter encoded by genes named ''Lsr'' (LuxS regulated). P''lsr'' is the promoter of the ''lsr'' operon. In our project, we use it to trigger the expression of a reporter gene. |
===Usage and Biology=== | ===Usage and Biology=== | ||
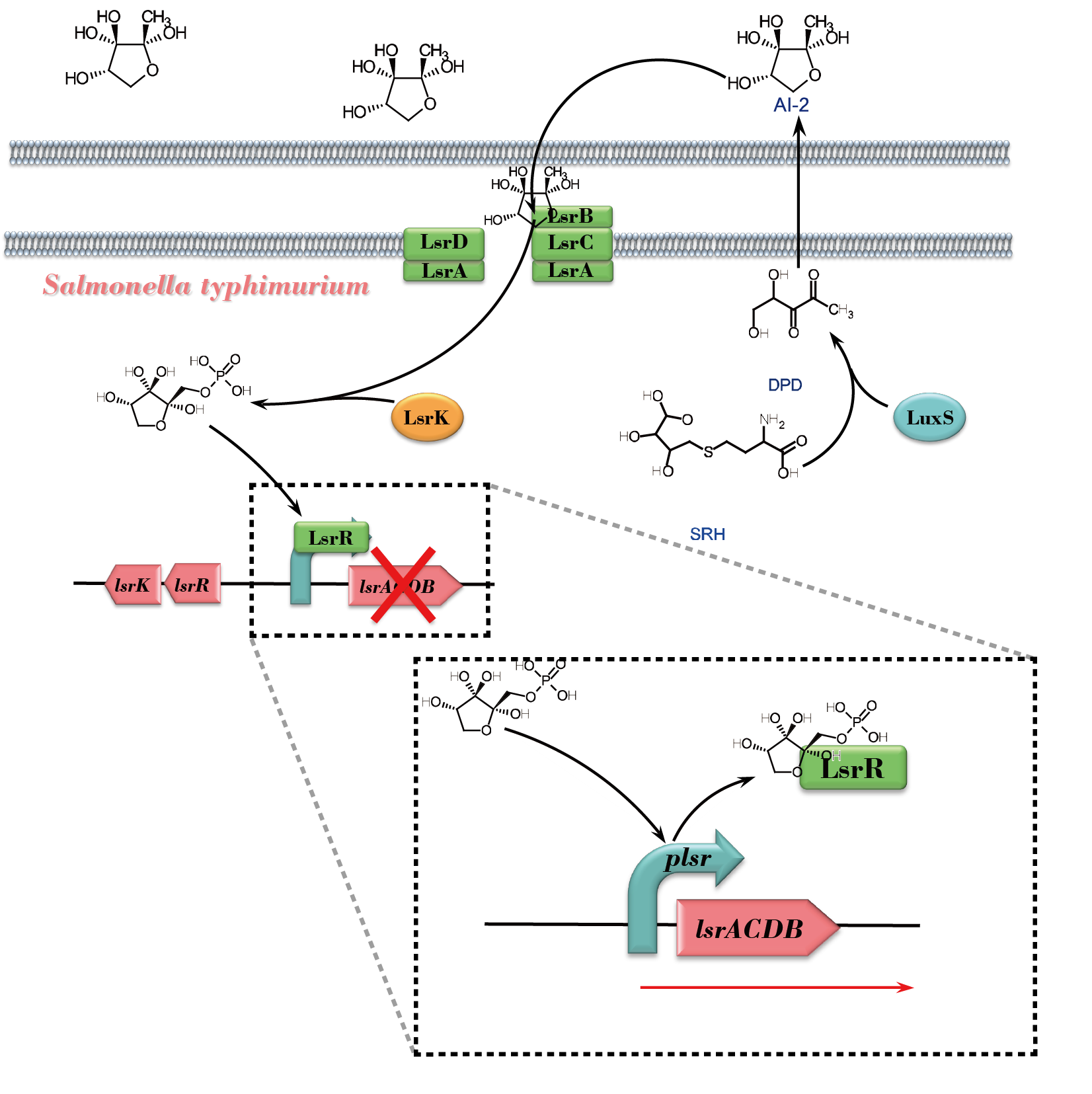
| − | AI-2 is generated by many species of Gram-negative and Gram-positive bacteria. In a group of bacteria exemplified by ''Salmonella'', AI-2 response involves ''lsr'' genes that encode ATP binding cassette-type transporter. | + | AI-2 is generated by many species of Gram-negative and Gram-positive bacteria. In a group of bacteria exemplified by ''Salmonella'', AI-2 response involves ''lsr'' genes that encode ATP binding cassette-type transporter. P''lsr'' is the promoter of the ''lsr'' operon. |
[[File:NEFU_China_2015_AI-2_response_in_Salmonalla.png|550px|thumb|center|'''Fig1. Schematic overview of the AI-2 response pathway in ''Salmonella Typhimurium'''''The precursor of AI-2, 4,5-Dihydroxy-2,3-Pentanedione (DPD) , is a byproduct generated when LuxS converts S-Ribosylhomocysteine (SRH) to Homocysteine (HCY). DPD then undergoes spontaneously cyclization, forming AI-2, and exports to the culture supernatant. After that, extracellular AI-2 bounds to LsrB, following by passing the membrane channel and importing the cytoplasm. LsrK phosphorylates AI-2 afterwards. The ''lsr'' operon is repressed until phosphorylated AI-2 causes LsrR to relieve its repression on the promoter. And this allows further AI-2 import.]] | [[File:NEFU_China_2015_AI-2_response_in_Salmonalla.png|550px|thumb|center|'''Fig1. Schematic overview of the AI-2 response pathway in ''Salmonella Typhimurium'''''The precursor of AI-2, 4,5-Dihydroxy-2,3-Pentanedione (DPD) , is a byproduct generated when LuxS converts S-Ribosylhomocysteine (SRH) to Homocysteine (HCY). DPD then undergoes spontaneously cyclization, forming AI-2, and exports to the culture supernatant. After that, extracellular AI-2 bounds to LsrB, following by passing the membrane channel and importing the cytoplasm. LsrK phosphorylates AI-2 afterwards. The ''lsr'' operon is repressed until phosphorylated AI-2 causes LsrR to relieve its repression on the promoter. And this allows further AI-2 import.]] | ||
| − | In our project, we | + | P''lsr'' is an inducible promoter under the repressive regulation of LsrR. In our project, we use it to regulate the expression of the reporter gene, ''amilCP'' (BBa_K592009).When pathogenic bacteria express AI-2 molecules and secrete them extracellularly, these AI-2 molecules can be transported into our engineered bacteria and trigger the expression of the report gene to produce the blue pigment. |
| − | [[File:NEFU_China_2015_Plsr_Blue.png|450px|thumb|center|'''Fig2. Part of the vector containing '' | + | [[File:NEFU_China_2015_Plsr_Blue.png|450px|thumb|center|'''Fig2. Part of the vector containing P''lsr'' ''']] |
===Characterization=== | ===Characterization=== | ||
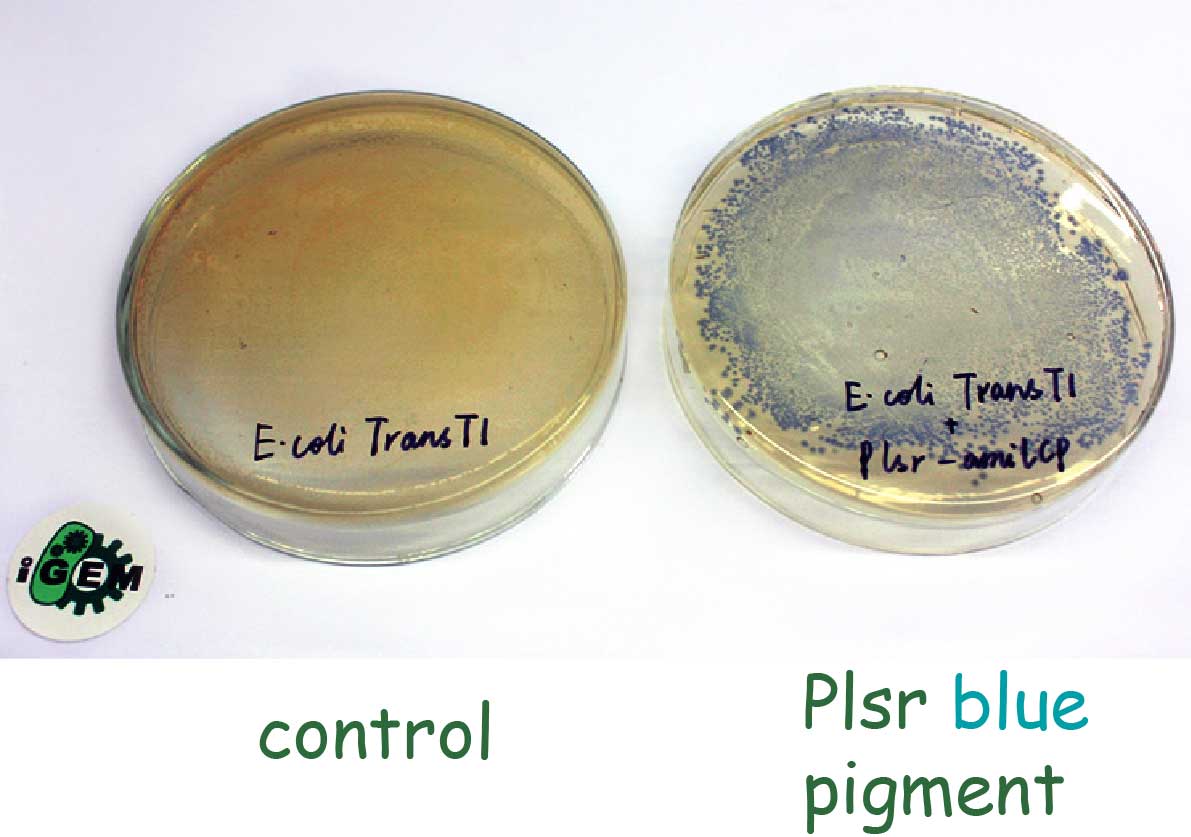
| − | We isolated the promoter region of the lsr operon to regulate the expression of the report gene. In order to test the function of the | + | We isolated the promoter region of the ''lsr'' operon to regulate the expression of the report gene, ''amilCP'' (BBa_K592009). In order to test the function of the p''lsr'' promoter, we transformed the plasmid pHY300PLK containing P''lsr'' with a blue pigment gene at its downstream into ''E. coli'' Trans T1 primarily. |
| − | + | As we can see, after overnight incubation, we observed blue colonies on the plate. That means without LsrR-mediated repression, the P''lsr'' promoter will be constitutively active and promote the production of visible blue pigment. | |
[[File:NEFU_China_2015_Ecoli_Blue.png|500px|thumb|center|'''Fig3. Engineered ''E. coli'' Trans T1 contains pHY300PLK-P''lsr''-''amilCP'' ''']] | [[File:NEFU_China_2015_Ecoli_Blue.png|500px|thumb|center|'''Fig3. Engineered ''E. coli'' Trans T1 contains pHY300PLK-P''lsr''-''amilCP'' ''']] | ||
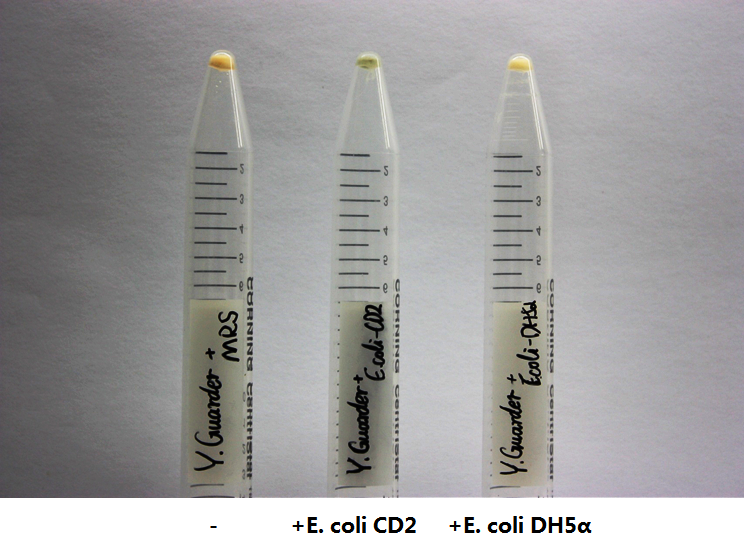
| − | After we have successfully integrated pNZ9530 and the expression vectors for ''lsrB'', ''R'' and ''K'' into the ''Lactobacillus'' genome, we sequentially transformed the P''lsr''- | + | After we have successfully integrated pNZ9530 and the expression vectors for ''lsrB'', ''R'' and ''K'' into the ''Lactobacillus'' genome, we sequentially transformed the P''lsr''-''amilCP'' vector. |
| − | We cultured our engineered Lactobacillus in the medium containing AI-2 secreted by E. coli CD-2. We used DH5alpha bacteria as a control because they do not produce any AI-2. Our engineered Lactobacillus showed clear blue color compared to the control. | + | We cultured our engineered ''Lactobacillus'' in the medium containing AI-2 secreted by ''E. coli'' CD-2. We used DH5alpha bacteria as a control because they do not produce any AI-2. Our engineered ''Lactobacillus'' showed clear blue color compared to the control. |
| − | + | The P''lsr'' was repressed unless phosphorylated AI-2 relieved the repression. This suggested the P''lsr'' promoter can indeed work as expected. | |
| − | [[File:NEFU_China_2015_Yogurt_Blue.png|500px|thumb|center|'''Fig4. | + | [[File:NEFU_China_2015_Yogurt_Blue.png|500px|thumb|center|'''Fig4. Engineered ''Lactobacillus'' incubated with culture supernatant of different bacteria ''']] |
Latest revision as of 03:55, 19 September 2015
lsr promoter of LuxS/AI-2 signaling pathway in Salmonalla
Quorum sensing is a process of bacterial cell-to-cell communication involving the production and detection of extracellular signaling molecules called autoinducers. And autoinducer-2 (AI-2) has been proposed to serve as a 'universal signal' for interspecies communication. In the LuxS/AI-2 signaling system of Salmonella Typhimurium, AI-2 response involves ATP binding cassette transporter encoded by genes named Lsr (LuxS regulated). Plsr is the promoter of the lsr operon. In our project, we use it to trigger the expression of a reporter gene.
Usage and Biology
AI-2 is generated by many species of Gram-negative and Gram-positive bacteria. In a group of bacteria exemplified by Salmonella, AI-2 response involves lsr genes that encode ATP binding cassette-type transporter. Plsr is the promoter of the lsr operon.
Plsr is an inducible promoter under the repressive regulation of LsrR. In our project, we use it to regulate the expression of the reporter gene, amilCP (BBa_K592009).When pathogenic bacteria express AI-2 molecules and secrete them extracellularly, these AI-2 molecules can be transported into our engineered bacteria and trigger the expression of the report gene to produce the blue pigment.
Characterization
We isolated the promoter region of the lsr operon to regulate the expression of the report gene, amilCP (BBa_K592009). In order to test the function of the plsr promoter, we transformed the plasmid pHY300PLK containing Plsr with a blue pigment gene at its downstream into E. coli Trans T1 primarily. As we can see, after overnight incubation, we observed blue colonies on the plate. That means without LsrR-mediated repression, the Plsr promoter will be constitutively active and promote the production of visible blue pigment.
After we have successfully integrated pNZ9530 and the expression vectors for lsrB, R and K into the Lactobacillus genome, we sequentially transformed the Plsr-amilCP vector. We cultured our engineered Lactobacillus in the medium containing AI-2 secreted by E. coli CD-2. We used DH5alpha bacteria as a control because they do not produce any AI-2. Our engineered Lactobacillus showed clear blue color compared to the control. The Plsr was repressed unless phosphorylated AI-2 relieved the repression. This suggested the Plsr promoter can indeed work as expected.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]